Magnaporthe grisea: Difference between revisions
imported>Chris Day |
imported>Chris Day |
||
| Line 26: | Line 26: | ||
==Genome structure== | ==Genome structure== | ||
Due to the destructive nature of this pathogen, sequencing the entire genome was necessary to understand the mechanisms of how the fungus causes disease. The complete genome of ''M.grisea'' was sequenced in 2005. ''M. grisea'' is a haploid and consists of ~40 Mb that are contained in 7 | Due to the destructive nature of this pathogen, sequencing the entire [[genome]] was necessary to understand the mechanisms of how the fungus causes disease. The complete genome of ''M.grisea'' was sequenced in 2005. ''M. grisea'' is a [[haploid]] and consists of ~40 Mb that are contained in 7 [[chromosome]]s.<ref>Talbot et al. 1993</ref> <ref>Orbach 1996</ref> The genome consists of a varied group of secreted proteins and a range of [[G-protein coupled receptor]] (GPCR) genes. Furthermore, strong phylogenetic evidence of [[horizontal gene transfer]] (HGT) has been found between ''M.grisea'' ([[ascomycete]] fungi) and [[oomycete]]s ( a distant relative). | ||
==Cell structure and metabolism== | ==Cell structure and metabolism== | ||
Revision as of 09:47, 17 April 2009
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is May 21, 2009. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! |
| Magnaporthe grisea
A conidium and conidiogenous cell of M. grisea | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Magnaporthe grisea |
Description and significance
Magnaporthe grisea, a plant-pathogenic fungus, is the causal agent of rice blast disease which is one of the greatest pathological threats to rice crops. It was thought to be localized only in developing nations, however for the past decade it has emerged as a severe problem in the United States, more recently in California. Yearly, rice that can feed an estimated 60 million people is destroyed by this disease. M.grisea is a well adapted fungus that can attack and penetrate its host plant with ease. More importantly, this fungus can also infect other important agricultures such as cereals, wheat, rye, pearl millet, and barley. The disease that is cause is then referred as blight disease or blast disease. Furthermore, M.grisea is thought of as a model organism in studies of fungal phytopathogenicity and host-parasite interactions.
Genome structure
Due to the destructive nature of this pathogen, sequencing the entire genome was necessary to understand the mechanisms of how the fungus causes disease. The complete genome of M.grisea was sequenced in 2005. M. grisea is a haploid and consists of ~40 Mb that are contained in 7 chromosomes.[1] [2] The genome consists of a varied group of secreted proteins and a range of G-protein coupled receptor (GPCR) genes. Furthermore, strong phylogenetic evidence of horizontal gene transfer (HGT) has been found between M.grisea (ascomycete fungi) and oomycetes ( a distant relative).
Cell structure and metabolism
Magnaporthe grisea is a filamentous fungi. The spore tips contains a STM(Spore Tip Mucilage) adhesive that allows M.grisea to bind to its host. The binding of the spore begins the infection progression. After host-binding, specialized infection cells termed appresoria provide mechanical force and thus penetrates the plant cuticle and so gain entry into the internal tissues of the host.
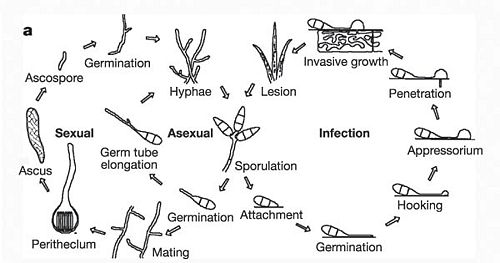
M.grisea are infectious species and so obtain nutrients from their hosts. M.grisea is haploid and can reproduce sexually and asexually. M.grisea infectiious life cycle is asexual. The conidium is the infectious structure in this life cycle. After colonization of its host, lesions form. The lesions sites are locations of M.grisea sporulation. Soon enough, nearby plants become infected as well and so the spread of rice blast disease.
Ecology
M.grisea inhibits the host plant’s immune system, affecting the metabolism and cell signaling pathways. Adaptation of M.grisea is so efficient that even rice cultivars (bred to resist the disease) are infected. All above-ground parts of a host plant can be infected, but only if the stem, node, or panicle becomes infected will the grain set be lost. Rice is one of the most important staple food worldwide and control of this disease is utmost important.
Life Cycle
Pathology
M.grisea produces conidia spores that become airborne and so land on rice plants. The spores are adhesive and stick to the plant. Germination is then followed and a specialized structure called the appresorium penetrates the plant surface. High nitrogen content, host plant moisture, and 20°C of night temperatures favors the disease. There are many races of this fungal pathogen and their level of virulence varies. Rice cultivars have been bred with the different resistance genes that have been identified, however the pathogen overcomes the resistance genes within a few years.
Control Measures
Burning/composting of diseased tissues
Diseased plants are burned or composted to prevent the spread of the disease to the next crop season.
Healthy Crop
For a healthy crop, healthy seeds are necessary and many precautions are to be taken. The seeds are collected from the field where M.grisea existence is unlikely due to hostile conditions for the pathogen. Fungicide and disinfectants are then applied if necessary for protection against a variety of pathogens.
Fertilizer management
Excess nitrogen fertilizer can facilitate the disease progression, whereas silica limits the development of the disease. The amount and type of fertilizer are to be discretionally applied.
Chemical control
The following fungicides are applied to combat the rice blast disease: benomyl, fthalide, edifenphos, iprobenfos, tricyclazole, isoprothiolane, probenazole, pyroquilon, felimzone(= meferimzone), diclocymet, carpropamid, fenoxanil and metominostrobin, and antibiotics such as blasticidin and kasugamycin.
Resistant cultivars
Based on studies and current knowledge of the different races of M.grisea, resistant cultivars have been bred throughout the world.
Current Research
The Genome Sequence of the Rice Blast Fungus Magnaporthe Grisea -This study was conducted to sequence the whole genome of M.grisea. Presented in this paper are preliminary analyses of the genome. The genome was sequenced via a shotgun approach. The draft genome sequenced consists of 2.273 sequence contigs that are longer than 2kbs. Total length of the genome is estimated to be ~40Mb. 11,109 genes are predicted to yield protein products and make up 48% of M.grisea genome. Evidently, phylogenitically none of the gene families of M.grisea shows recent lineage-specific expansion indicating that ancient gene duplication may have played a role followed by loss of the gene families in the other lineages (see Fig.S1) .It is found that M.grisea has a considerable amount of proteins that are diverse and novel in cell pathways. Furthermore, the generation of genetic variations are tremendous and could explain the efficiency of this fungal pathogen to infect resistant rice cultivars within a few generations.