Circadian rhythms and appetite: Difference between revisions
imported>Fiona E Graham |
imported>Laura Sheldon |
||
| Line 71: | Line 71: | ||
Peripheral clocks | Peripheral clocks | ||
The SCN and peripheral clocks are not affected by meal timings, but restriction of food is the dominant synchroniser for peripheral clocks. When rats are restricted of food, metabolic and hormonal factors used by the SCN to drive peripheral oscillators are uncoupled, to shift to food time. Normally, the SCN and peripheral oscillators will work together as one unit, but a change in food availability can uncouple them in order for survival, when feeding is low and shifted from their normal place in the light-dark cycle.<ref>Escobar C ''et al.'' (2009) Peripheral oscillators are important for food anticipatory activity (FAA)'''' </ref>. | The SCN and peripheral clocks are not affected by meal timings, but restriction of food is the dominant synchroniser for peripheral clocks. When rats are restricted of food, metabolic and hormonal factors used by the SCN to drive peripheral oscillators are uncoupled, to shift to food time. Normally, the SCN and peripheral oscillators will work together as one unit, but a change in food availability can uncouple them in order for survival, when feeding is low and shifted from their normal place in the light-dark cycle.<ref>Escobar C ''et al.'' (2009) Peripheral oscillators are important for food anticipatory activity (FAA)European Journal of Neuroscience, 30: 1665–1675 '''' </ref>. | ||
The Food Entrained Oscillator | The Food Entrained Oscillator | ||
| Line 81: | Line 81: | ||
[[User:Laura Sheldon|Laura Sheldon]] 19:09, 24 October 2010 (UTC)Laura | [[User:Laura Sheldon|Laura Sheldon]] 19:09, 24 October 2010 (UTC)Laura | ||
==Sleep deprivation, shift-work and appetite== | ==Sleep deprivation, shift-work and appetite== | ||
Revision as of 05:38, 26 October 2010
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is 01 February 2011. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! |
Begin your article with a brief overview of the scope of the article on interest group. Include the article name in bold in the first sentence.[1]
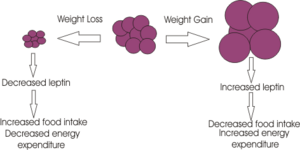
Remember you are writing an encyclopedia article; it is meant to be readable by a wide audience, and so you will need to explain some things clearly, without using unneccessary jargon. But you don't need to explain everything - you can link specialist terms to other articles about them - for example adipocyte or leptin simply by enclosing the word in double square brackets.
You can write your article directly onto the wiki- but at first you'll find it easier to write it in Word and copy and paste it onto the wiki.
Construct your article in sections and subsections, with headings and subheadings like this:
Introduction
The control of food intake is a flexible system whereby internal and external environmental cues can alter the timing of feeding and appetite. The suprachiasmatic nucleus in the hypothalamus is the vital coordinator of these stimuli that ultimately generates fluctuations neuronal and hormonal activities known as circadian rhythms.
Circadian rhythms are driven by the light and dark cycles of the earth which through alteration of gene expression elicit a host of physiological responses including fluctuations in the hormones involved in appetite and food intake.
A wide variety of organisms, from cyanobacteria to humans, all share common internal clock mechanisms that have been present for millions of years in evolutionary history. The circadian clock in mammals is responsible for setting specific temporal patterns within our bodily systems, such as general physiological functions (body temperature, melatonin release, glucocorticoid secretion) and behavioural functions (alertness, working memory,) in order to keep us alive and running smoothly.
The "master" circadian clock of mammals is found in the suprachiasmatic nucleus (SCN) of the hypothalamus. Circadian signals from the SCN are distributed by diffusible/humoral messages and by neuronal outputs [2]. These signals influence clocks found in peripheral tissues (liver, kidney, thymus, muscle –Guillaumend et al 2005). Other brain regions express clock genes with self-sustained oscillations (retina, olfactory bulb and striatum), but these don’t have clocks.
Clock mechanisms are formed by the transcription and translation of clock genes, which rely on feedback loops. In mammals, Clock and Bmal1 genes are part of the positive loop and Per genes, the negative loop. The clock genes regulate a self-sustaining rhythm in cells that even without light will maintain a roughly 24 hour rhythm. An extreme example is of cave fish which have lived in complete darkness for millions of years and their clock genes are still present in their DNA.[3].
Rhythmic output of organs can be influenced by metabolic, endocrine and homeostatic events, as well as the circadian clock. For example, the SCN can change the rhythm of liver genes and enzymes without using clock genes, but through 2nd messenger systems induced by the ANS instead. Also other genes can have effect on circadian clock genes, for example ROR-alpha gene is a positive regulator of Bmal1 gene, which regulates lipogenesis and lipid storage (Lau et. Al 2004).
It has been well documented that food intake itself can regulate these rhythms and yet the neural mechanisms by which this occurs remains elusive. It has recently been proposed that there exists a ‘food entrainable oscillator’ that exists independently from the SCN which controls food anticipation activity.
Various factors such as have been known to influence circadian rhythms indeed our modern day lifestyle has highlighted new factors that influence these delicate balances including jet-lag and shift work.
SCN, the biological clock
Suprachiasmatic Nucleus
Suprachiasmatic nucleus of the anterior hypothalamus is the main regulator of circadian rhythms. SCN is made of neuronal and glial cells, with most of the neurons being GABAergic. Main input into SCN is light. Light is transmitted from retina via the retinohypothalamic tract to SCN. (Froy, 2010) Vasoactive intrinsic polypeptide in SCN activates and synchronises SCN neurons and coordinates behavioural rhythms. SCN signals to peripheral oscillators using the following actors: TGFα, prokinecticin 2 and cardiotrophin like cytokine and neuronal connections to prevent dampening of circadian rhythms in the tissues. (Froy, 2010) SCN efferent fibres terminate around the arcuate nucleus of ventromedial hypothalamus (VMH) and paraventricular nucleus PVN, areas involved in regulation of food intake and corticosteroid release. (Froy, 2010) SCN innervates sub-paraventricular zone (SPZ) and dorsomedial hypothalamus (DMH) which in-turn innervate PVN and lateral hypothalamus, areas which regulate corticosteroid release and wakefulness / feeding cycle. (Froy, 2010) DMH regulates sleep-wakefulness and feeding cycles among others and degeneration of DMH results in severe impairment of those cycles. (Froy, 2010) SCN selectively innervates preautonomic nervous system neurons found in dorsal and ventral borders of PVN.As pre-autonomic neurons in the hypothalamus are connected to sympathetic and parasympathetic system, this allows SCN to control energy homeostasis. (Froy, 2010) SCN receives information on hormones and metabolites, which are present in the bloodstream and cannot cross blood-brain barrier, via its dense reciprocal interactions with ventromedial arcuate nucleus (vmARC). vmARC receives the above information through its connection with circumventricular median eminence. Circumventricular median eminence is a structure within the brain which is free of blood-brain barrier and hormones can easily reach their receptors on neurons. Gastrin-releasing peptide interacts with SCN and results in light-like resetting of SCN. (Froy, 2010)
~~Image Figure 3. SCN afferents and efferents. The SCN can be activated by light, hormones and nutrients, neuronal connections (green arrows).The SCN neuronal connections to the ARC, MPOA, PVN and SPZ (blue arrows) ARC is affected by hormones and nutrients directly. SPZ innervates the DMH, with DMH innervating PVN, VLPO and LH which coordinate corticosteroid production, sleep, feeding respectively. PVN and DMH regulate adipose tissue, liver and other peripheral tissues through autonomic nervous system (red arrows).
Clock Genes
~~ Genes encoding core clock mechanism are circadian locomotor output cycles kaput (Clock), brain and muscle-Arnt-like 1(Bmal1), Period1 (Per1),Period2 (Per2), Period3 (Per3),Cryptochrome1(Cry1) and Cryptochrome2(Cry2). (Froy 2010). CLOCK transcription factor dimerises with BMAL1 and activates transcription. CLOCK and BMAL1 are basic helix-loop-helix-PAS transcription factors which upon binding to E-box and E-box like promoter sequences activate transcription. The action of CLOCK:BMAL1 heterodimer is inhibited by PER and CRY proteins. Products of Clock gene are important in regulating appetite. Mice whose Clock function was impaired had an increased food intake and rhythmic expression of Cart and Orexin hormones was eradicated(Froy 2010). Experimental data reported by Bray et.al shows that CLOCK -/- mice exhibit obesity,altered feeding patterns,hyperphagia and hormonal abnormalities similar to those found in metabolic syndromes, such as hyperlipidemia, hyperleptinemia, hyperglycemia and hyperinsulinemia.
SCN & Feeding Time
The main way in which the SCN regulates food intake is by directing the sleep/wake cycle. The role of the SCN in feeding patterns has been determined using rodents studies as unlike humans their food intake is less influenced by cognition and social behaviour. The sleep/wake cycle is driven by fluctuations in two main hormones: corticosterone (cortisol in humans) and melatonin. Corticosterone levels rise during the night when the nocturnal animals active (). Their rise is followed by an increase in activity in which they forage for food and subsequently begin to feed. Melatonin is an important hormone that is released from the pineal gland during the night (the day in noturnal animals) which amongst its many actions induces sleep and suppresses appetite ().
PICTURE OF SCN CONTROLLING CORTISONE AND MELATONIN RELEASE Figure Legend: Glucocorticoids: GABAergic projections from the SCN inhibit neuronal activity in the PVN during the day [4]. In the night these inhibitory signals are suppressed and glutamatergic neurons stimulate CRH release from the PVN. CRH in the hypothalamic-portal system then triggers the release of ACTH from anterior pituitary into the blood stream that ultimately causes the release of glucocorticoids from the adrenal cortex. Melatonin: The SCN is connected to the pineal gland by a multisynaptic pathway which successively includes neurons of the paraventricular nucleus of the hypothalamus and a number of other brain regions. The release of noradrenaline into the pineal gland during the night stimulates the melatonin synthesis pathway. The study by Perreau et al. (2004) found that the rhythm of melatonin synthesis is formed by a combination of inhibitory and stimulatory signals from the SCN to the PVN and that the SCN-derived glutamate release within the PVN is the main stimulus for melatonin synthesis.
Whilst the SCN is known to influence feeding patterns indirectly by regulating the awake/sleep cycles, it remains unclear whether or not the SCN can directly drive appetite. As the SCN has reciprocal interactions with the orexigenic regions of the brain it is possible that the SCN can directly stimulate appetite.
PICTURE : It has been found that SCN fibres terminate in and around the arcuate nucleus (ARC) and the ventral part of the lateral hypothalamus (Yi et al. 2006). Activation of the arcuate nucleus releases NYP and AGRP (two potent orexigenic peptides) into the PVN which ultimately stimulates feeding and slows metabolism maximising energy intake [5]. However, research into this possibility is yet to be carried out.
Alternatively, it is possible that the SCN initiates feeding by conducting circadian rhythmic oscillations in the hormones involved in appetite. Indeed it has recently been established that a number of hormones involved in feeding behaviour and appetite, including leptin, ghrelin, and orexins show circadian oscillations. (Reviwed by Froy 2010). GRAPHS Leptin has been shown to exhibit circadian patterns in both gene expression and protein secretion in humans, with a peak during the sleep phase in humans (Kalra et al. 2003 in froy). Furthermore, rodent studies have shown that ablation of the SCN eliminates leptin circadian rhythmicity (Kalsbeek 2001 in Froy) and yet the role of the SCN in conducting this pattern is unclear. As leptin binds to receptors in the hypothalamus to suppress of appetite and an increase metabolism (Schwartz et al. 2000) it seems plausible to suggest that the SCN can alter appetite indirectly via hormone regulation.
Conclusion…
Fiona E Graham 15:57, 25 October 2010 (UTC)
Peripheral Clocks and Food Entrainable Oscillators
Peripheral clocks
The SCN and peripheral clocks are not affected by meal timings, but restriction of food is the dominant synchroniser for peripheral clocks. When rats are restricted of food, metabolic and hormonal factors used by the SCN to drive peripheral oscillators are uncoupled, to shift to food time. Normally, the SCN and peripheral oscillators will work together as one unit, but a change in food availability can uncouple them in order for survival, when feeding is low and shifted from their normal place in the light-dark cycle.[6].
The Food Entrained Oscillator
The FEO is a mysterious circadian clock, which is independent of the SCN. It ensures that when food is scarce, the body is still ready to digest and extract nutrients from the food that has been found, the FEO is responsible for anticipation of meal-time (FAA) [7].
Clock genes may contribute to the FAA but are not essential – Pendergast et al (2009) showed that animals without essential clock genes Bmal1 or per1 or2 were arrhythmic in constant darkness but still could express FAA. The FEO’s existence is putative, and its network is believed to be scattered over several brain regions. For example, the dorsomedial hypothalamic nucleus has been reported for FAA expression and the possible site of the FEO. The circadian mechanism for the FEO is unknown, but it does present clear circadian features.
Laura Sheldon 19:09, 24 October 2010 (UTC)Laura
Sleep deprivation, shift-work and appetite
In modern society, where shopping, eating, working and drinking are widely available 24hours a day,
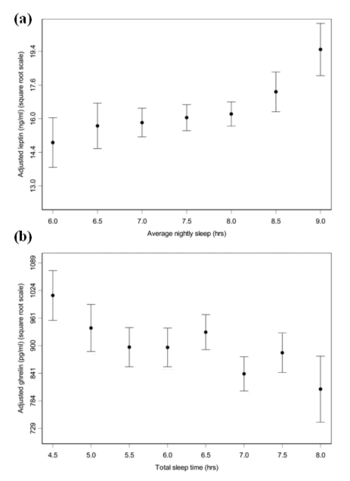
Figure 5.1. The relationship between sleep duration and changes in serum leptin and ghrelin levels. (a) Mean leptin levels against average nightly sleep duration. As the number of hours sleep increases the levels of serum leptin also increases. Standard errors for half-hour increments of average nightly sleep. (b) Mean ghrelin levels against total number of hours sleep. As the total number of hours sleep decreases, the mean levels of ghrelin increases. Standard errors for half-hour increments of total sleep time. This figure has been adapted from Taheri et. al (2004).
major health implications have been linked as a result. This availability of around the clock activities has defied our bodies internal clock of the vital hours of sleep it requires [8]. Over the last few decades where technology and social activities has dramatically advanced, the number of hours of sleep young adults get has decreased within the range of 1-2hours [9]. This is strongly correlated with the prevalence of obesity within the U.K which has shown to have trebled over the last 3 decades [10]. Although the rise in this obesity epidemic has been shown to be multi-factorial, sleep deprivation is just another factor to add to the list needing to be addressed in this ever rising health problem.
Several epidemiologic studies have shown that sleep deprivation elevates the levels of the appetite stimulating hormone ghrelin and decreases circulating leptin levels [Fig. 5.1] [11][9]. These changes could be the causes of increased food intake in these sleep- deprived adults where a rise in body weight is also observed [Fig. 5.2] [11].
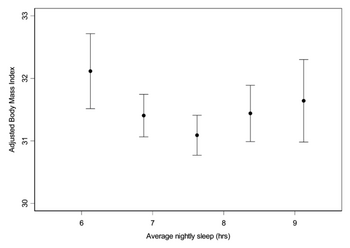
Conclusion
etc.
About References
To insert references and/or footnotes in an article, put the material you want in the reference or footnote between <ref> and </ref>, like this:
<ref>Person A ''et al.''(2010) The perfect reference for subpart 1 ''J Neuroendocrinol'' 36:36-52</ref> <ref>Author A, Author B (2009) Another perfect reference ''J Neuroendocrinol'' 25:262-9</ref>.
Look at the reference list below to see how this will look.[12] [13]
If there are more than two authors just put the first author followed by et al. (Person A at al. (2010) etc.)
Select your references carefully - make sure they are cited accurately, and pay attention to the precise formatting style of the references. Your references should be available on PubMed and so will have a PubMed number. (for example PMID: 17011504) Writing this without the colon, (i.e. just writing PMID 17011504) will automatically insert a link to the abstract on PubMed (see the reference to Johnsone et al. in the list.)
[14]
Use references sparingly; there's no need to reference every single point, and often a good review will cover several points. However sometimes you will need to use the same reference more than once.
How to write the same reference twice:
Reference: Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431 PMID 17072591
First time: <ref name=Berridge07>Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. ''Psychopharmacology'' 191:391–431 PMID 17072591 </ref>
Second time:<ref name=Berridge07/>
This will appear like this the first time [15] and like this the second time [15]
Figures and Diagrams
You can also insert diagrams or photographs (to Upload files Cz:Upload)). These must be your own original work - and you will therefore be the copyright holder; of course they may be based on or adapted from diagrams produced by others - in which case this must be declared clearly, and the source of the orinal idea must be cited. When you insert a figure or diagram into your article you will be asked to fill out a form in which you declare that you are the copyright holder and that you are willing to allow your work to be freely used by others - choose the "Release to the Public Domain" option when you come to that page of the form.
When you upload your file, give it a short descriptive name, like "Adipocyte.png". Then, if you type {{Image|Adipocyte.png|right|300px|}} in your article, the image will appear on the right hand side.
References
- ↑ See the "Writing an Encyclopedia Article" handout for more details.
- ↑ Kalsbeek et al.(2007) Minireview: circadian control of metabolism by the suprachiasmatic nuclei Endocrinology 148:5635-9
- ↑ Mendoza J, Challet E (2009) Brain clocks: From the suprachiasmatic nuclei to a cerebral network The Neuroscientist 15: 5
- ↑ Feillet CA (2010)) Food for Thoughts:Feeding Time and Hormonal Secretion. Journal of Neuroendocrinology 22:620-628.
- ↑ Schwartz MW (2007)Central nervous system control of food intake. Nature 404:661-671
- ↑ Escobar C et al. (2009) Peripheral oscillators are important for food anticipatory activity (FAA)European Journal of Neuroscience, 30: 1665–1675 '
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedMendoza09 - ↑ Gimble JM et al. (2009) Circadian biology and sleep: missing links in obesity and metabolism? Obesity Rev 10(suppl 2):1-5.
- ↑ 9.0 9.1 Spiegel et. al(2004)Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite Ann Internal Med 141:846-50
- ↑ Rennie KL, Jebb SA (2005) Prevalence of obesity in Great Britain Obesity Rev 6:11-2
- ↑ 11.0 11.1 Taheri et al. (2004) Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Medicine 1(3):e62
- ↑ Person A et al. (2010) The perfect reference for subpart 1 J Neuroendocrinol 36:36-52
- ↑ Author A, Author B (2009) Another perfect reference J Neuroendocrinol 25:262-9
- ↑ Johnstone LE et al. (2006)Neuronal activation in the hypothalamus and brainstem during feeding in rats Cell Metab 2006 4:313-21. PMID 17011504
- ↑ 15.0 15.1 Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431 PMID 17072591