Air stripping: Difference between revisions
imported>Milton Beychok m (Expanded the lede section a bit.) |
mNo edit summary |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
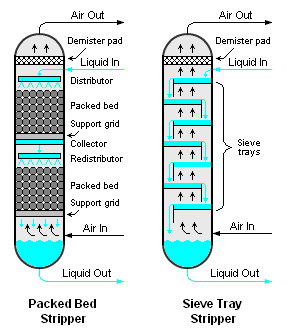
{{Image|Air Strippers.png|right|287px|Figure 1: Diagram of two different types of air strippers}} | {{Image|Air Strippers.png|right|287px|Figure 1: Diagram of two different types of air strippers}} | ||
'''Air stripping''' is the transferring of ''volatile organic compounds'' (VOC) and/or other [[Volatility (chemistry)|volatile]] components of a liquid into an [[air]] stream. It is a [[chemical engineering]] process used for the purification of [[groundwater]]s and [[wastewater]]s containing [[Volatility|volatile]] compounds.<ref name=Sellers>{{cite book|author=Kathleen Sellers|title=Hazardous Waste Site Remediation|edition=1st Edition|publisher=CRC Press|year=1998|pages=pages 131 - 134|ID=ISBN 1-56670-281-X}}</ref><ref name=Jaeger>[http://www.jaeger.com/Brochure/airstripping.pdf Air Stripping of VOCs from Water] From the website of Jaeger Products, Inc.</ref> | '''Air stripping''' is the transferring of ''volatile organic compounds'' (VOC) and/or other [[Volatility (chemistry)|volatile]] components of a liquid into an [[air]] stream. It is a [[chemical engineering]] process used for the purification of [[groundwater]]s and [[wastewater]]s containing [[Volatility|volatile]] compounds.<ref name=Sellers>{{cite book|author=Kathleen Sellers|title=Hazardous Waste Site Remediation|edition=1st Edition|publisher=CRC Press|year=1998|pages=pages 131 - 134|ID=ISBN 1-56670-281-X}}</ref><ref name=Jaeger>[http://www.jaeger.com/Brochure/airstripping.pdf Air Stripping of VOCs from Water] From the website of Jaeger Products, Inc.</ref> | ||
| Line 9: | Line 8: | ||
The compounds removed by air stripping include [[benzene]], [[toluene]], [[ethylbenzene]], and [[xylene]] (BTEX) and various solvents (such as [[trichloroethylene]] and [[tetrachloroethylene]]) found in some wastewaters. [[Ammonia]] can also be stripped from wastewaters. | The compounds removed by air stripping include [[benzene]], [[toluene]], [[ethylbenzene]], and [[xylene]] (BTEX) and various solvents (such as [[trichloroethylene]] and [[tetrachloroethylene]]) found in some wastewaters. [[Ammonia]] can also be stripped from wastewaters. | ||
There are many methods for the air stripping of volatile components from liquids, but the most common methods are the use of either a [[Packed bed|packed]] or [[Theoretical tray|trayed]] vertical cylindrical vessel <ref name=Seader>{{cite book|author=J.D. Seader and E.J. Henley|title=Separation Process Principles|edition=2nd Edition|publisher=John Wiley & Sons|year=2006|isbn=0-471-46480-5}}</ref><ref>{{cite book|author=P. Chattpadhyay|title=Absorption and Stripping|edition=1st Edition|publisher=Asian Books Pvt.Ltd.|year=2007|pages=pages 2.98 - 2.116|id=ISBN 81-8412-033-8}}</ref><ref>{{cite book|author=Edward E. Baruth (Technical Editor)|title=Water Treatment Plant Design|edition=4th Edition|publisher=American Water Works Association and American Society of Civil Engineers|year=2004|id=ISBN 0-07-141872-5}} See chapter 5.</ref> as shown in Figure 1, and this article devoted primarily to those two methods. | There are many methods for the air stripping of volatile components from liquids, but the most common methods are the use of either a [[Packed bed|packed]] or [[Theoretical tray|trayed]] vertical cylindrical vessel <ref name=Seader>{{cite book|author=J.D. Seader and E.J. Henley|title=Separation Process Principles|edition=2nd Edition|publisher=John Wiley & Sons|year=2006|isbn=0-471-46480-5}}</ref><ref>{{cite book|author=P. Chattpadhyay|title=Absorption and Stripping|edition=1st Edition|publisher=Asian Books Pvt.Ltd.|year=2007|pages=pages 2.98 - 2.116|id=ISBN 81-8412-033-8}}</ref><ref>{{cite book|author=Edward E. Baruth (Technical Editor)|title=Water Treatment Plant Design|edition=4th Edition|publisher=American Water Works Association and American Society of Civil Engineers|year=2004|id=ISBN 0-07-141872-5}} See chapter 5.</ref> as shown in Figure 1, and this article is devoted primarily to those two methods. | ||
== Process description == | == Process description == | ||
{{Image|Koch-Glitsch Cascade Rings.jpg|right|180px|Types of randomly dumped packing.}} | |||
{{Image|Koch-Glitsch Structured Packing.jpg|right|180px|An example of structured packing.}} | |||
Either the packed or trayed air stripper involves the downward flow of a volatile-containing liquid (most often, water) and the upward flow of air. The liquid and air are intimately contacted by the packing or the trays in the stripper. The volatile components in the liquid are transferred into the air stream by the series of intimate contacts occurring at each tray as the air flows upward through the stripper. | Either the packed or trayed air stripper involves the downward flow of a volatile-containing liquid (most often, water) and the upward flow of air. The liquid and air are intimately contacted by the packing or the trays in the stripper. The volatile components in the liquid are transferred into the air stream by the series of intimate contacts occurring at each tray as the air flows upward through the stripper. | ||
| Line 17: | Line 18: | ||
A properly designed and operated air stripper can remove up to 99.9+ percent of volatile organic compounds (VOC) from groundwaters and wastewaters. | A properly designed and operated air stripper can remove up to 99.9+ percent of volatile organic compounds (VOC) from groundwaters and wastewaters. | ||
There are many different types of trays, including bubble-cap trays, valve | There are many different types of trays, including bubble-cap trays, valve trays and sieve trays.<ref>'''Note:''' Sieve trays are simply circular metal plates perforated with many holes through which the air flows upward to come into intimate contact with the liquid flowing across the plates (see Figure 1). Valve trays are similar to sieve trays except that the perforations are covered with caps that are free to move upward. See the [[Theoretical plate]] article for a drawing and explanation of bubble-cap trays.</ref> Sieve trays are the most commonly used type in air strippers because they are very simple, less expensive than the other types and adequately efficient for use in air strippers. There are also many types of packing available for [[packed bed]] strippers, either randomly packed or structurally packed material as shown in the adjacent photographs. | ||
The stripped liquid leaving the bottom of the stripper is essentially free of volatiles. The air stream leaving the top of the stripper contains the volatiles removed from the liquid and may require some type of [[Air pollution emissions|emissions]] mitigation to comply with applicable environmental regulatory limits such as the | The stripped liquid leaving the bottom of the stripper is essentially free of volatiles. The air stream leaving the top of the stripper contains the volatiles removed from the liquid and may require some type of [[Air pollution emissions|emissions]] mitigation to comply with applicable environmental regulatory limits such as the | ||
| Line 27: | Line 28: | ||
* [[Catalytic oxidation]] | * [[Catalytic oxidation]] | ||
* [[Incineration]] (also known as ''thermal oxidation'') | * [[Incineration]] (also known as ''thermal oxidation'') | ||
If utilized in a large complex such as a petroleum refinery or petrochemical plant, then the air emissions from a stripper may be used as combustion air in one of the many fired heaters (i.e., process furnaces) used in such complexes. In some such cases, the VOC combustion products would only be [[carbon dioxide]] and water. | |||
The relative effectiveness of the above mitigation technologies depend to a great extent on the concentration level of the pollutants in the air stream from an air stripper. Very low pollutant concentrations mean that very large volumes of air must be handled in order to mitigate very small amounts of pollutants. GAC filtration is generally used most frequently because it has the ability to remove VOC cost-effectively from air stream containing less than 1% of VOC. | The relative effectiveness of the above mitigation technologies depend to a great extent on the concentration level of the pollutants in the air stream from an air stripper. Very low pollutant concentrations mean that very large volumes of air must be handled in order to mitigate very small amounts of pollutants. GAC filtration is generally used most frequently because it has the ability to remove VOC cost-effectively from air stream containing less than 1% of VOC. | ||
In some cases, | In some cases, [[air pollution dispersion modeling]] studies may be used to determine whether or not routing the air stream through a vent stack of a reasonable height would result in compliance with the applicable environmental regulations without requiring any mitigation of the air pollutant emissions from the air stripping system. | ||
Figure 2 (below) is a simplified, schematic flow diagram of a complete air stripping system including optional provisions for heating the feed water: | Figure 2 (below) is a simplified, schematic flow diagram of a complete air stripping system including optional provisions for heating the feed water: | ||
{{Image|Air Stripping System.png|center|410px|Figure 2: Schematic flow diagram of a complete air stripping system, including optional heating of the feed water.}} | {{Image|Air Stripping System.png|center|410px|Figure 2: Schematic flow diagram of a complete air stripping system, including optional heating of the feed water.}} | ||
== Design variables == | == Design variables == | ||
| Line 42: | Line 45: | ||
The selection of whether to use a packed or trayed air stripper is primarily based on which is more cost effective for each specific application. However, in general: | The selection of whether to use a packed or trayed air stripper is primarily based on which is more cost effective for each specific application. However, in general: | ||
*If the liquid contains dispersed solids, the trays in a trayed stripper are easily accessible for cleaning. By contrast, the packing in a packed stripper would have to be completely removed to be cleaned or acid washed in place. | |||
*If the liquid contains dispersed solids, the trays in a trayed stripper are easily accessible for cleaning. By contrast, the packing in a packed stripper would have to be completely removed to be cleaned. | |||
*Trayed strippers are seldom designed with cross-sectional diameters less than 60 [[Centimetre|cm]] (about 2 [[Foot|feet]]). | *Trayed strippers are seldom designed with cross-sectional diameters less than 60 [[Centimetre|cm]] (about 2 [[Foot|feet]]). | ||
*Large diameter packed strippers require very careful design of the liquid flow distributor and redistributors (see Figure 1) to avoid flow channelling which reduces performance efficiency. | |||
*Packed strippers are preferred for liquids having a tendency to foam since that interferes significantly with the efficiency of trayed strippers. | *Packed strippers are preferred for liquids having a tendency to foam since that interferes significantly with the efficiency of trayed strippers. | ||
*Packed | *Packed stripperss are generally less expensive. They are also easier to construct if the liquid is highly corrosive. | ||
* | *Trayed strippers have a lower profile (shorter heights and smaller diameter) than an equivalent packed stripper. | ||
*Trayed strippers can use valve trays rather than sieve trays to provide a lower turndown capability than an equivalent packed stripper. | |||
=== Other important design variables === | === Other important design variables === | ||
| Line 59: | Line 63: | ||
* The limits set by the applicable governmental air pollution regulations as to the pollutants contained in the exit air from the stripping system. | * The limits set by the applicable governmental air pollution regulations as to the pollutants contained in the exit air from the stripping system. | ||
* The temperature of the entering liquid. | * The temperature of the entering liquid. | ||
* Higher operating temperatures make it easier to remove volatile components from the entering liquid. Thus, consideration should be given to the cost effectiveness of heating | * Higher operating temperatures make it easier to remove volatile components from the entering liquid. Thus, consideration should be given to the cost effectiveness of heating the entering liquid to the maximum temperature that can be used without vaporizing any of the liquid. | ||
* For packed to towers, the selection of the type of packing to be used. Especially, whether to use randomly dumped packing or structured packing (see the above two photographs). | * For packed to towers, the selection of the type of packing to be used. Especially, whether to use randomly dumped packing or structured packing (see the above two photographs). | ||
| Line 91: | Line 95: | ||
The sole focus of this article is to discuss the air stripping process. However, gases other than air can be used to strip volatile components from liquids. For example, steam and inert gases (such as nitrogen) have been and are being used for stripping liquids of undesirable, volatile components. | The sole focus of this article is to discuss the air stripping process. However, gases other than air can be used to strip volatile components from liquids. For example, steam and inert gases (such as nitrogen) have been and are being used for stripping liquids of undesirable, volatile components. | ||
== Other applications == | |||
Air stripping is used for a number of applications other than removing VOCs from ground water and from wastewaters. In the chemical process industries and elsewhere, air stripping is used for dechlorination of water, denitrification of water, and the removal of gases such as [[ammonia]], [[carbon dioxide]] and [[hydrogen sulphide]] from water. | |||
The well-known [[Selexol]] process uses a physical solvent (propylene glycol dimethyl ether) to absorb and remove [[acid gas]]es such as hydrogen sulfide and/or carbon dioxide from gas streams. The solvent is then stripped of the acid gases by using either steam or air stripping and, in some cases, partially stripped by steam and partially stripped by air.<ref name=Kohl>{{cite book|author=Arthur Kohl and Richard Nielson|title=Gas Purification|edition=5th Edition|publisher=Gulf Publishing|year=1997|id=ISBN 0-88415-220-0}}</ref> The process has been effectively used for carbon dioxide removal in a number of of [[ammonia synthesis]] production plants as well as in some [[natural gas processing]] plants. | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}}[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 12:01, 7 July 2024
Air stripping is the transferring of volatile organic compounds (VOC) and/or other volatile components of a liquid into an air stream. It is a chemical engineering process used for the purification of groundwaters and wastewaters containing volatile compounds.[1][2]
Air stripping for the removal of contaminants from groundwaters and wastewaters is especially effective for contaminants that have a high vapor pressure and a low solubility inwater.[1][2]
The compounds removed by air stripping include benzene, toluene, ethylbenzene, and xylene (BTEX) and various solvents (such as trichloroethylene and tetrachloroethylene) found in some wastewaters. Ammonia can also be stripped from wastewaters.
There are many methods for the air stripping of volatile components from liquids, but the most common methods are the use of either a packed or trayed vertical cylindrical vessel [3][4][5] as shown in Figure 1, and this article is devoted primarily to those two methods.
Process description
Either the packed or trayed air stripper involves the downward flow of a volatile-containing liquid (most often, water) and the upward flow of air. The liquid and air are intimately contacted by the packing or the trays in the stripper. The volatile components in the liquid are transferred into the air stream by the series of intimate contacts occurring at each tray as the air flows upward through the stripper.
A properly designed and operated air stripper can remove up to 99.9+ percent of volatile organic compounds (VOC) from groundwaters and wastewaters.
There are many different types of trays, including bubble-cap trays, valve trays and sieve trays.[6] Sieve trays are the most commonly used type in air strippers because they are very simple, less expensive than the other types and adequately efficient for use in air strippers. There are also many types of packing available for packed bed strippers, either randomly packed or structurally packed material as shown in the adjacent photographs.
The stripped liquid leaving the bottom of the stripper is essentially free of volatiles. The air stream leaving the top of the stripper contains the volatiles removed from the liquid and may require some type of emissions mitigation to comply with applicable environmental regulatory limits such as the National Emissions Standards for Hazardous Air Pollutants (HAP) as set by the U.S. Environmental Protection Agency.
When mitigation of the air pollutant emissions from an air stripper is required, there are three technologies available:
- Adsorption in granular activated carbon filters (also known as GAC filters).
- Catalytic oxidation
- Incineration (also known as thermal oxidation)
If utilized in a large complex such as a petroleum refinery or petrochemical plant, then the air emissions from a stripper may be used as combustion air in one of the many fired heaters (i.e., process furnaces) used in such complexes. In some such cases, the VOC combustion products would only be carbon dioxide and water.
The relative effectiveness of the above mitigation technologies depend to a great extent on the concentration level of the pollutants in the air stream from an air stripper. Very low pollutant concentrations mean that very large volumes of air must be handled in order to mitigate very small amounts of pollutants. GAC filtration is generally used most frequently because it has the ability to remove VOC cost-effectively from air stream containing less than 1% of VOC.
In some cases, air pollution dispersion modeling studies may be used to determine whether or not routing the air stream through a vent stack of a reasonable height would result in compliance with the applicable environmental regulations without requiring any mitigation of the air pollutant emissions from the air stripping system.
Figure 2 (below) is a simplified, schematic flow diagram of a complete air stripping system including optional provisions for heating the feed water:
Design variables
Selection of the air stripper type
The selection of whether to use a packed or trayed air stripper is primarily based on which is more cost effective for each specific application. However, in general:
- If the liquid contains dispersed solids, the trays in a trayed stripper are easily accessible for cleaning. By contrast, the packing in a packed stripper would have to be completely removed to be cleaned or acid washed in place.
- Trayed strippers are seldom designed with cross-sectional diameters less than 60 cm (about 2 feet).
- Large diameter packed strippers require very careful design of the liquid flow distributor and redistributors (see Figure 1) to avoid flow channelling which reduces performance efficiency.
- Packed strippers are preferred for liquids having a tendency to foam since that interferes significantly with the efficiency of trayed strippers.
- Packed stripperss are generally less expensive. They are also easier to construct if the liquid is highly corrosive.
- Trayed strippers have a lower profile (shorter heights and smaller diameter) than an equivalent packed stripper.
- Trayed strippers can use valve trays rather than sieve trays to provide a lower turndown capability than an equivalent packed stripper.
Other important design variables
The are many variables to be considered in the design of an air stripping system. Among them are:
- The volumetric flow rate of the liquid entering the air stripping system.
- The specific volatile components to be removed from the liquid and their concentrations in the liquid entering the stripping system as well as their chemical and physical properties (such as vapor pressure, solubility in the liquid, whether or not they are highly toxic or hazardous, and others).
- The physical properties of the liquid (such as the vapor pressures and boiling points over a range of temperatures, viscosity, corrosivity, and others).
- The percentage removal of volatile components from the liquid needed to meet the requirements of any downstream processing of the stripped liquid or to comply with any applicable governmental regulatory requirements for the ultimate disposal of the stripped liquid.
- The limits set by the applicable governmental air pollution regulations as to the pollutants contained in the exit air from the stripping system.
- The temperature of the entering liquid.
- Higher operating temperatures make it easier to remove volatile components from the entering liquid. Thus, consideration should be given to the cost effectiveness of heating the entering liquid to the maximum temperature that can be used without vaporizing any of the liquid.
- For packed to towers, the selection of the type of packing to be used. Especially, whether to use randomly dumped packing or structured packing (see the above two photographs).
Typical performance data
VOC removal performance data collected from 46 operating air strippers in the United States are summarized in the table below:[7]
| VOC | % Removal |
|---|---|
| Benzene | 99 to 99.9 |
| Toluene | 96 to 99+ |
| Xylene | 96 to 99.8 |
| Trichloroethylene | 90 to 99.9 |
| Methyl-tert-butyl ether (MEK) | 95 to 99 |
| 2-Methy phenol * | 70+ |
| Phenol * | 74 |
| Aniline * | 58 |
| Note: * Only one data point | |
Other stripping gases
The sole focus of this article is to discuss the air stripping process. However, gases other than air can be used to strip volatile components from liquids. For example, steam and inert gases (such as nitrogen) have been and are being used for stripping liquids of undesirable, volatile components.
Other applications
Air stripping is used for a number of applications other than removing VOCs from ground water and from wastewaters. In the chemical process industries and elsewhere, air stripping is used for dechlorination of water, denitrification of water, and the removal of gases such as ammonia, carbon dioxide and hydrogen sulphide from water.
The well-known Selexol process uses a physical solvent (propylene glycol dimethyl ether) to absorb and remove acid gases such as hydrogen sulfide and/or carbon dioxide from gas streams. The solvent is then stripped of the acid gases by using either steam or air stripping and, in some cases, partially stripped by steam and partially stripped by air.[8] The process has been effectively used for carbon dioxide removal in a number of of ammonia synthesis production plants as well as in some natural gas processing plants.
References
- ↑ 1.0 1.1 Kathleen Sellers (1998). Hazardous Waste Site Remediation, 1st Edition. CRC Press, pages 131 - 134.
- ↑ 2.0 2.1 Air Stripping of VOCs from Water From the website of Jaeger Products, Inc.
- ↑ J.D. Seader and E.J. Henley (2006). Separation Process Principles, 2nd Edition. John Wiley & Sons. ISBN 0-471-46480-5.
- ↑ P. Chattpadhyay (2007). Absorption and Stripping, 1st Edition. Asian Books Pvt.Ltd., pages 2.98 - 2.116. ISBN 81-8412-033-8.
- ↑ Edward E. Baruth (Technical Editor) (2004). Water Treatment Plant Design, 4th Edition. American Water Works Association and American Society of Civil Engineers. ISBN 0-07-141872-5. See chapter 5.
- ↑ Note: Sieve trays are simply circular metal plates perforated with many holes through which the air flows upward to come into intimate contact with the liquid flowing across the plates (see Figure 1). Valve trays are similar to sieve trays except that the perforations are covered with caps that are free to move upward. See the Theoretical plate article for a drawing and explanation of bubble-cap trays.
- ↑ Ralph Stephenson and James B. Blackburn (1997). The Industrial Wastewater Systems Handbook, 1st Edition. CRC Press. ISBN 1-56670-209-7.
- ↑ Arthur Kohl and Richard Nielson (1997). Gas Purification, 5th Edition. Gulf Publishing. ISBN 0-88415-220-0.