Glucose: Difference between revisions
imported>David E. Volk No edit summary |
mNo edit summary |
||
| (6 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
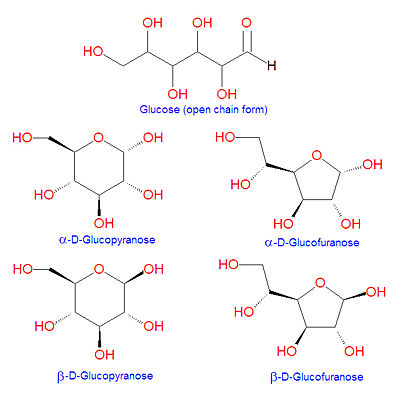
{{Image|Glucose structures.jpg|right|400px|Different forms of glucose}} | |||
'''Glucose''' is a [[hexose]] [[sugar]] molecule present in living organisms. It is the main form of energy in a cell, and the first step of [[ | '''Glucose''' is a [[hexose]] [[sugar]] molecule present in living organisms. It is the main form of energy in a cell, and the first step of [[glycolysis]] is the transformation of glucose to [[glucose-6-phosphate]]. Glucose is also the precursor or the major antioxidant and enzyme cofactor, [[vitamin C]], in most animals (except [[human]]s, great apes and a few other species). It is a component of the [[disaccharide]]s [[sucrose]], [[galactose]] and [[maltose]], to name a few. Because it contains an [[aldehyde]] in the open chain structure, it is one of the [[aldose]] sugars. Glucose can cyclize to form five- and six-atom ring structures called [[glucofuranose]] and [[glucopyranose]], respectively, as shown in the illustration. In 1891, the German chemist [[Emil Fischer]] elucidated the structure of D-glucose. | ||
== Cyclization mechanism == | == Cyclization mechanism == | ||
Glucose can undergo [[intramolecular reaction]]s in which the aldehyde carbon at position C-1 is subject to nucleophilic attack by either the C-4 or C-5 hydroxyl groups to form cyclic [[hemiacetal]] structures called [[glucofuranose]] or [[glucopyranose]], respectively. Depending on the stereochemical orientation of the resulting C-1 hydroxyl group formed in this reaction, the products are labeled as alpha or beta (see illustration). | Glucose can undergo [[intramolecular reaction]]s in which the aldehyde carbon at position C-1 is subject to nucleophilic attack by either the C-4 or C-5 hydroxyl groups to form cyclic [[hemiacetal]] structures called [[glucofuranose]] or [[glucopyranose]], respectively. Depending on the stereochemical orientation of the resulting C-1 hydroxyl group formed in this reaction, the products are labeled as alpha or beta (see illustration).[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:01, 22 August 2024
Glucose is a hexose sugar molecule present in living organisms. It is the main form of energy in a cell, and the first step of glycolysis is the transformation of glucose to glucose-6-phosphate. Glucose is also the precursor or the major antioxidant and enzyme cofactor, vitamin C, in most animals (except humans, great apes and a few other species). It is a component of the disaccharides sucrose, galactose and maltose, to name a few. Because it contains an aldehyde in the open chain structure, it is one of the aldose sugars. Glucose can cyclize to form five- and six-atom ring structures called glucofuranose and glucopyranose, respectively, as shown in the illustration. In 1891, the German chemist Emil Fischer elucidated the structure of D-glucose.
Cyclization mechanism
Glucose can undergo intramolecular reactions in which the aldehyde carbon at position C-1 is subject to nucleophilic attack by either the C-4 or C-5 hydroxyl groups to form cyclic hemiacetal structures called glucofuranose or glucopyranose, respectively. Depending on the stereochemical orientation of the resulting C-1 hydroxyl group formed in this reaction, the products are labeled as alpha or beta (see illustration).