Hetacillin: Difference between revisions
imported>David E. Volk (stub and structure, more to follow tomorrow) |
mNo edit summary |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
[[Image:Hetacilliln structure.jpg| | |||
{{Chem infobox | |||
|align=right | |||
|image=[[Image:Hetacilliln structure.jpg|center|thumb|250px|{{#ifexist:Template:Hetacilliln structure.jpg/credit|{{Hetacilliln structure.jpg/credit}}<br/>|}}]] | |||
|width=250px | |||
|molname=hetacillin | |||
|synonyms= | |||
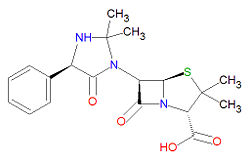
|molformula= C<sub>19</sub>H<sub>23</sub>N<sub>3</sub>O<sub>4</sub>S | |||
|molmass= 389.4686 | |||
|uses=antibiotic drug | |||
|properties=beta-lactam | |||
|hazards=see drug interactions | |||
|iupac= see chemistry section | |||
|casnumber=3511-16-8 | |||
}} | |||
'''Hetacillin''' is a [[penicillin]]-like, beta-[[lactam]] based [[antibiotic]] | |||
'''Hetacillin''' is a [[penicillin]]-like, beta-[[lactam]]-based [[antibiotic]] prodrug used to treat infections, usually from [[Gram-positive]] bacteria. It is sold under the brand names Hetacillin potassium®, Versapen® and Versapen-k®. | |||
== Mechanism of action == | |||
Hetacillin is a prodrug with no antibacterial activity, but it is metabolized into the antibiotic [[ampicillin]]. Hetacillin is prepared by reacting ampicillin with acetone because ampicillin is much less stable towards ring-opening reactions. Once converted to ampicillin, the ampicillin interferes with the final stage of cell wall synthesis by binding to penicillin-binding proteins, leading to [[autolysis]] of the bacteria by [[autolysin]] enzymes. | |||
== Chemistry == | |||
It is a beta-[[lactam]] structure, and its chemical name is (2S,5R,6R)-6-[(4R)-2,2-dimethyl-5-oxo-4-phenylimidazolidin-1-yl]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid. Its chemical formula is C<sub>19</sub>H<sub>23</sub>N<sub>3</sub>O<sub>4</sub>S (MW = 389.4686 g/mol). Hetacillin is susceptible to degradation in bacteria that produce [[beta-lactamase]]. | |||
== External links == | |||
{{CZMed}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 16:00, 27 August 2024
|
| |||||||
| hetacillin | |||||||
| |||||||
| Uses: | antibiotic drug | ||||||
| Properties: | beta-lactam | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Hetacillin is a penicillin-like, beta-lactam-based antibiotic prodrug used to treat infections, usually from Gram-positive bacteria. It is sold under the brand names Hetacillin potassium®, Versapen® and Versapen-k®.
Mechanism of action
Hetacillin is a prodrug with no antibacterial activity, but it is metabolized into the antibiotic ampicillin. Hetacillin is prepared by reacting ampicillin with acetone because ampicillin is much less stable towards ring-opening reactions. Once converted to ampicillin, the ampicillin interferes with the final stage of cell wall synthesis by binding to penicillin-binding proteins, leading to autolysis of the bacteria by autolysin enzymes.
Chemistry
It is a beta-lactam structure, and its chemical name is (2S,5R,6R)-6-[(4R)-2,2-dimethyl-5-oxo-4-phenylimidazolidin-1-yl]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid. Its chemical formula is C19H23N3O4S (MW = 389.4686 g/mol). Hetacillin is susceptible to degradation in bacteria that produce beta-lactamase.
External links
The most up-to-date information about Hetacillin and other drugs can be found at the following sites.
- Hetacillin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Hetacillin - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Hetacillin - Detailed information from DrugBank.