Insulin: Difference between revisions
imported>Douglas Robert Kelly mNo edit summary |
mNo edit summary |
||
| (28 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{TOC|right}} | |||
In [[physiology]], the hormone '''Insulin''' is the key regulator of blood [[glucose]] levels and is released from specialized [[Insulin-secreting cell|beta cells]] within the [[Islets of Langerhans]] of the endocrine [[pancreas|pancreas]]. Whilst the Islets comprise only a small percentage of the pancreas (1-2 %), they serve the major physiological function in [[homeostasis|homeostatic]] control of blood glucose concentrations and cellular metabolism. The [[Insulin-secreting cell|beta cells]] of the pancreas release stored insulin in response to a range of neural, nutrient, hormonal and chemical stimuli but primarily in response to increased concentrations of glucose. Insulin secretion is increased by high blood glucose levels and inhibited by falling glucose levels. The overall effect of insulin is to stimulate anabolic (energy storage) processes to lower blood levels of glucose, [[fatty acid]]s, and [[amino acid]]s and to promote their conversion to the respective storage forms of [[glycogen]], triglycerides and protein.<br /> | |||
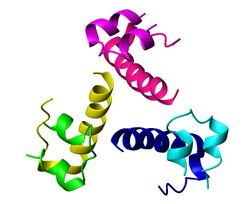
{{Image|Insulin.jpg|right|250px|X-ray crystal structure of human insulin.<ref>Coordinates determined by Norrman, Hubalek and Schluckebier (2007) Structural characterization of insulin NPH formulations. Eur.J.Pharm.Sci. 30: 414-423.</ref>}} | |||
==Mechanism of secretion == | ==Mechanism of secretion == | ||
'''Primary mechanism of Insulin secretion by the pancreas'''<ref>http://www.medscape.com/viewarticle/438368</ref> | '''Primary mechanism of Insulin secretion by the pancreas'''<ref>http://www.medscape.com/viewarticle/438368</ref> | ||
: | : | ||
* Glucose enters beta cells of the | * Glucose enters [[Insulin-secreting cell|beta cells]] of the Islets of Langerhans by facilitated diffusion involving GLUT-2 glucose transporters.<br /> | ||
* This cytosolic glucose is metabolized to ATP increasing the intracellular ratio of ATP:ADP. <br /> | * This cytosolic glucose is metabolized to ATP increasing the intracellular ratio of ATP:ADP. <br /> | ||
* Elevation in the intracellular ATP concentration induces closure of cell-surface ATP-sensitive K<sup>+</sup> (K<sub>ATP</sub>) channels. This reduces repolarizing current density leading to cell membrane depolarization.<br /> | * Elevation in the intracellular ATP concentration induces closure of cell-surface ATP-sensitive K<sup>+</sup> (K<sub>ATP</sub>) channels. This reduces repolarizing current density leading to cell membrane depolarization.<br /> | ||
* Voltage-dependent Ca<sup>2+</sup> channels open in response to membrane depolarization, facilitating movement of extracellular Ca<sup>2+</sup> into the beta cell. <br /> | * Voltage-dependent Ca<sup>2+</sup> channels open in response to membrane depolarization, facilitating movement of extracellular Ca<sup>2+</sup> into the beta cell. <br /> | ||
* The rising cytosolic Ca<sup>2+</sup> triggers | * Increasing cytosolic calcium concentrations activate phospholipase C stimulating the production of inositol 1,4,5-triphosphate (IP<sub>3</sub>) and diacylglycerol (DAG) from membrane phospholipids. <br /> | ||
*IP<sub>3</sub> binds to intracellular receptors on the membrane of the endoplasmic reticulum (ER) initiating the release of large amounts of stored calcium into the cytoplasm<br /> | |||
* The rising cytosolic Ca<sup>2+</sup> triggers exocytosis of insulin containing vesicles within the beta cell into nearby blood vessels. The extensive vascularization of the pancreatic islets region ensures rapid diffusion of insulin into blood circulation and subsequent delivery to target cells and tissues. | |||
==In diabetes== | ==In diabetes== | ||
| Line 20: | Line 22: | ||
==Insulin Therapy== | ==Insulin Therapy== | ||
===Types of insulin=== | |||
Available insulin include human and pork derived with the latter infrequently used after human preparations were available. In addition, analogues of human insulin are available but have uncertain benefit.<ref name="pmid19221352">{{cite journal |author=Singh SR ''et al.'' |title=Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta-analysis |journal=CMAJ |volume=180 |pages=385–97 |year=2009 |month=February |pmid=19221352 |pmc=2638025 |doi=10.1503/cmaj.081041 |url=http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=19221352 |issn=}}</ref><ref name="pmid18794553">{{cite journal |author=Qayyum R ''et al.'' |title=Systematic review: comparative effectiveness and safety of premixed insulin analogues in type 2 diabetes |journal=Ann Intern Med |volume=149 |pages=549–59 |year=2008 |month=October |pmid=18794553 |doi= |url= |issn=}}</ref> | |||
The commonly used types of insulin are: | The commonly used types of insulin are: | ||
====Rapid-acting==== | |||
Rapid-acting insulins include three analogues:<ref name="pmid18936506">{{cite journal |author=Majumdar S, Barrett E |title=Newer insulins in search of a niche |journal=Annals of internal medicine |volume=149 |pages=586–8 |year=2008 |month=October |pmid=18936506 |doi= |url= |issn=}}</ref>Anonymous (2009)<ref>[http://www.medicalletter.org/restricted/articles/w1327b.html Rapid-Acting Insulin Analogs] The Medical Letter</ref> | |||
* insulin ''lispro'' – begins to work within 5 to 15 minutes and is active for 3 to 4 hours.<ref>{{CZMed|Insulin lispro}} | |||
</ref> | |||
* insulin aspart<ref>{{CZMed|Insulin aspart}} | |||
</ref> | |||
* insulin glulisine | |||
====Short-acting==== | |||
Short-acting insulins include ''regular'' insulin – starts working within 30 minutes and is active about 5 to 8 hours. | |||
====Intermediate-acting==== | |||
Intermediate-acting act within 1 to 3 hours and are active 16 to 24 hours. | |||
* neutral protamine Hagedorn (NPH) insulin | |||
* ''lente'' insulin | |||
* neutral protamine lispro (NPL) insulin | |||
====Long-acting==== | |||
Long-acting insulins include: | |||
* ''ultralente'' insulin – starts working in 4 to 6 hours, and is active 24 to 28 hours. | |||
* ''Insulin glargine'' (LANTUS™)<ref>{{CZMed|Insulin glargine}} | |||
</ref> – starts working within 1 to 2 hours and continue to be active, without major peaks or dips, for about 24 hours, although this varies in many individuals. | |||
* ''Insulin detemir'' (Levemir™)<ref>{{CZMed|Insulin detemir}} | |||
</ref> – starts working within 1 to 2 hours and continue to be active for about 24 hours, although typically not as long acting as glargine. | |||
A [[meta-analysis]] of [[randomized controlled trial]]s by the [[Cochrane Collaboration]] found "only a minor clinical benefit of treatment with long-acting insulin analogues for patients with diabetes mellitus type 2."<ref name="pmid17443605">{{cite journal |author=Horvath K ''et al.'' |title=Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus |journal=Cochrane database of systematic reviews (Online) |volume= |pages=CD005613 |year=2007 |pmid=17443605}}</ref> More recent [[randomized controlled trial]]s have found no differences with glargine<ref name="pmid18936501">{{cite journal |author=Esposito K ''et al.'' |title=Addition of neutral protamine lispro insulin or insulin glargine to oral type 2 diabetes regimens for patients with suboptimal glycemic control: a randomized trial |journal=Ann Intern Med |volume=149 |pages=531–9 |year=2008 |pmid=18936501 |doi= |url= |issn=}}</ref> and have found that although long acting insulins were less effective, they were associated with less hypoglycemia.<ref name="pmid17890232">{{cite journal |author=Holman RR ''et al.'' |title=Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes |journal=N Engl J Med |volume=357 |pages=1716–30 |year=2007 |pmid=17890232 |doi=10.1056/NEJMoa075392 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=17890232&promo=ONFLNS19 |issn=}}</ref> | |||
====Premixed insulins==== | |||
Mixtures can be of two types of human insulins or two insulin analogues. Human mixtures and analogue mixtures have similar efficacy.<ref name="pmid18794553" /> | |||
Mixtures of human insulin: | |||
* A mixture of NPH and regular insulin – starts working in 30 minutes and is active 16 to 24 hours. There are several variations with different proportions of the mixed insulins. | * A mixture of NPH and regular insulin – starts working in 30 minutes and is active 16 to 24 hours. There are several variations with different proportions of the mixed insulins. | ||
Mixtures of analogue insulin: | |||
* NPL/lispro 75/25 | |||
=== Modes of administration === | === Modes of administration === | ||
| Line 50: | Line 75: | ||
====Inhalation==== | ====Inhalation==== | ||
In | In 2006 the U.S. [[Food and Drug Administration]] approved the use of [[Exubera]], the first inhalable insulin.<ref>[http://www.fda.gov/bbs/topics/news/2006/NEW01304.html FDA approval of ''Exubera'' inhaled insulin]</ref> Inhaled insulin has similar efficacy to injected insulin, both in terms of controlling glucose levels and blood half-life. Currently, inhaled insulin is short acting and is typically taken before meals; an injection of long-acting insulin at night is often still required.<ref name=ACD/> When patients were switched from injected to inhaled insulin, no significant difference was found in Hb<sub>A1c</sub> levels over three months. Accurate dosing is still a problem, but patients showed no significant weight gain or pulmonary function decline over the length of the trial, when compared to the baseline.<ref>{{cite journal|author=Cefalu W, Skyler J, Kourides I, Landschulz W, Balagtas C, Cheng S, Gelfand R|title=Inhaled human insulin treatment in patients with type 2 diabetes mellitus|journal=Ann Intern Med|volume=134|issue=3|pages=203–7|year=2001|id=PMID 11177333}}</ref> | ||
Following its commercial launch in 2005 in the UK, it has not (as of July 2006) been recommended by [[National Institute for Health and Clinical Excellence]] for routine use, except in cases where there is ''"proven injection phobia diagnosed by a psychiatrist or psychologist"''.<!-- | Following its commercial launch in 2005 in the UK, it has not (as of July 2006) been recommended by [[National Institute for Health and Clinical Excellence]] for routine use, except in cases where there is ''"proven injection phobia diagnosed by a psychiatrist or psychologist"''.<!-- | ||
--><ref name=ACD>{{cite web | title=Diabetes (type 1 and 2), Inhaled Insulin - Appraisal Consultation Document (second) | url=http://www.nice.org.uk/page.aspx?o=332283 | year=2006 | month=June 21 | author=[[National Institute for Health and Clinical Excellence|NICE]] | accessdate=2006-07-26}}</ref> | --><ref name=ACD>{{cite web | title=Diabetes (type 1 and 2), Inhaled Insulin - Appraisal Consultation Document (second) | url=http://www.nice.org.uk/page.aspx?o=332283 | year=2006 | month=June 21 | author=[[National Institute for Health and Clinical Excellence|NICE]] | accessdate=2006-07-26}}</ref> | ||
| Line 73: | Line 98: | ||
====Artificial pancreas==== | ====Artificial pancreas==== | ||
==Adverse effects== | |||
There is conflicting evidence whether administration of exogenous insulin may increase cancer risk.<ref name="pmid19597799">{{cite journal |author=Smith U, Gale EA |title=Does diabetes therapy influence the risk of cancer? |journal=Diabetologia |volume=52 |issue=9 |pages=1699–708 |year=2009 |month=September |pmid=19597799 |doi=10.1007/s00125-009-1441-5 |url=http://dx.doi.org/10.1007/s00125-009-1441-5 |issn=}}</ref> The increased risk may be due to increased affinity of insulin analogues to [[insulin-like growth factor I]].<ref name="pmid10866053">{{cite journal| author=Kurtzhals P, Schäffer L, Sørensen A, Kristensen C, Jonassen I, Schmid C et al.| title=Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. | journal=Diabetes | year= 2000 | volume= 49 | issue= 6 | pages= 999-1005 | pmid=10866053 | |||
| url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&retmode=ref&cmd=prlinks&id=10866053 }} </ref> | |||
{| class="wikitable" | |||
|+ Studies of insulin glargine and cancer risk<ref name="pmid19565214">{{cite journal |author=Hemkens LG, Grouven U, Bender R, ''et al.'' |title=Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study |journal=Diabetologia |volume=52 |issue=9 |pages=1732–44 |year=2009 |month=September |pmid=19565214 |pmc=2723679 |doi=10.1007/s00125-009-1418-4 |url=http://dx.doi.org/10.1007/s00125-009-1418-4 |issn=}}</ref><ref name="pmid19588120">{{cite journal |author=Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steineck G |title=Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden |journal=Diabetologia |volume=52 |issue=9 |pages=1745–54 |year=2009 |month=September |pmid=19588120 |doi=10.1007/s00125-009-1444-2 |url=http://dx.doi.org/10.1007/s00125-009-1444-2 |issn=}}</ref><ref name="pmid19572116">{{cite journal |author=Currie CJ, Poole CD, Gale EA |title=The influence of glucose-lowering therapies on cancer risk in type 2 diabetes |journal=Diabetologia |volume=52 |issue=9 |pages=1766–77 |year=2009 |month=September |pmid=19572116 |doi=10.1007/s00125-009-1440-6 |url=http://dx.doi.org/10.1007/s00125-009-1440-6 |issn=}}</ref><ref name="pmid19603149">{{cite journal |author=Colhoun HM |title=Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group |journal=Diabetologia |volume=52 |issue=9 |pages=1755–65 |year=2009 |month=September |pmid=19603149 |pmc=2723678 |doi=10.1007/s00125-009-1453-1 |url=http://dx.doi.org/10.1007/s00125-009-1453-1 |issn=}}</ref><ref name="pmid19609501">{{cite journal |author=Rosenstock J, Fonseca V, McGill JB, ''et al.'' |title=Similar risk of malignancy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: findings from a 5 year randomised, open-label study |journal=Diabetologia |volume=52 |issue=9 |pages=1971–3 |year=2009 |month=September |pmid=19609501 |pmc=2723677 |doi=10.1007/s00125-009-1452-2 |url=http://dx.doi.org/10.1007/s00125-009-1452-2 |issn=}}</ref> | |||
! Study!! Study design!!Finding | |||
|- | |||
| German health insurance<ref name="pmid19565214"/> || [[cohort study]]<br/>• 127,031 patients<br/>• mean follow-up of 1.63 years|| "the [[cancer]] incidence with glargine was higher than expected compared with human insulin" | |||
|- | |||
| Sweden prescription database<ref name="pmid19588120"/> || [[cohort study]]<br/>• 114,841 patients<br/>• follow-up < 2.5 years|| "women using insulin glargine alone (no other types of insulin) had an increased incidence rate of [[breast cancer]] as compared with women using types of insulin other than insulin glargine." | |||
|- | |||
| UK general practices<ref name="pmid19572116"/> || [[cohort study]]<br/>• 62,809 patients<br/>• follow-up < 9 years|| "Those on insulin or insulin secretagogues were more likely to develop solid [[cancer]]s than those on metformin, and combination with metformin abolished most of this excess risk" | |||
|- | |||
| Scottish Diabetes Research Network<ref name="pmid19603149"/> || [[cohort study]]<br/>• 36,254 patients<br/>• follow-up 4 years|| "Overall, insulin glargine use was not associated with an increased risk of all cancers or site-specific cancers in Scotland over a 4 year time frame. Given the overall data, we consider the excess of cases of all cancers and [[breast cancer]] in the subgroup of insulin glargine only users to more likely reflect allocation bias rather than an effect of insulin glargine itself" | |||
|- | |||
| North American trial<ref name="pmid19609501"/> || [[randomized controlled trial]]<br/>• 1017 patients<br/>• follow-up 4 years|| "data reported here also confirm that there was no evidence of any difference in the rate of benign or malignant tumour development with insulin glargine compared with NPH insulin." | |||
|} | |||
In many patients, insulin is an appetite stimulant; it is sometimes used off-label specifically for that purpose. Like most diabetes treatments, other than metformin and some of the newer classes such as incretin analogues, it often causes weight gain. | |||
==References== | ==References== | ||
{{reflist|2}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 11:01, 1 September 2024
In physiology, the hormone Insulin is the key regulator of blood glucose levels and is released from specialized beta cells within the Islets of Langerhans of the endocrine pancreas. Whilst the Islets comprise only a small percentage of the pancreas (1-2 %), they serve the major physiological function in homeostatic control of blood glucose concentrations and cellular metabolism. The beta cells of the pancreas release stored insulin in response to a range of neural, nutrient, hormonal and chemical stimuli but primarily in response to increased concentrations of glucose. Insulin secretion is increased by high blood glucose levels and inhibited by falling glucose levels. The overall effect of insulin is to stimulate anabolic (energy storage) processes to lower blood levels of glucose, fatty acids, and amino acids and to promote their conversion to the respective storage forms of glycogen, triglycerides and protein.
Mechanism of secretion
Primary mechanism of Insulin secretion by the pancreas[2]
- Glucose enters beta cells of the Islets of Langerhans by facilitated diffusion involving GLUT-2 glucose transporters.
- This cytosolic glucose is metabolized to ATP increasing the intracellular ratio of ATP:ADP.
- Elevation in the intracellular ATP concentration induces closure of cell-surface ATP-sensitive K+ (KATP) channels. This reduces repolarizing current density leading to cell membrane depolarization.
- Voltage-dependent Ca2+ channels open in response to membrane depolarization, facilitating movement of extracellular Ca2+ into the beta cell.
- Increasing cytosolic calcium concentrations activate phospholipase C stimulating the production of inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) from membrane phospholipids.
- IP3 binds to intracellular receptors on the membrane of the endoplasmic reticulum (ER) initiating the release of large amounts of stored calcium into the cytoplasm
- The rising cytosolic Ca2+ triggers exocytosis of insulin containing vesicles within the beta cell into nearby blood vessels. The extensive vascularization of the pancreatic islets region ensures rapid diffusion of insulin into blood circulation and subsequent delivery to target cells and tissues.
In diabetes
- Main Article: Diabetes mellitus
Patients suffering from diabetes mellitus have abnormal insulin function. In Type-I (or Juvenile), the pancreas produces insufficient amounts of insulin, resulting in high blood sugar levels. In Type-II diabetes, insulin receptors on cells become less responsive to insulin in the blood and there is a general alteration in the way cells respond to this hormone. Thus, while the pancreas may be producing enough insulin, the hormonal signal does not get through, resulting in high blood sugar levels.
Insulin Therapy
Types of insulin
Available insulin include human and pork derived with the latter infrequently used after human preparations were available. In addition, analogues of human insulin are available but have uncertain benefit.[3][4]
The commonly used types of insulin are:
Rapid-acting
Rapid-acting insulins include three analogues:[5]Anonymous (2009)[6]
- insulin lispro – begins to work within 5 to 15 minutes and is active for 3 to 4 hours.[7]
- insulin aspart[8]
- insulin glulisine
Short-acting
Short-acting insulins include regular insulin – starts working within 30 minutes and is active about 5 to 8 hours.
Intermediate-acting
Intermediate-acting act within 1 to 3 hours and are active 16 to 24 hours.
- neutral protamine Hagedorn (NPH) insulin
- lente insulin
- neutral protamine lispro (NPL) insulin
Long-acting
Long-acting insulins include:
- ultralente insulin – starts working in 4 to 6 hours, and is active 24 to 28 hours.
- Insulin glargine (LANTUS™)[9] – starts working within 1 to 2 hours and continue to be active, without major peaks or dips, for about 24 hours, although this varies in many individuals.
- Insulin detemir (Levemir™)[10] – starts working within 1 to 2 hours and continue to be active for about 24 hours, although typically not as long acting as glargine.
A meta-analysis of randomized controlled trials by the Cochrane Collaboration found "only a minor clinical benefit of treatment with long-acting insulin analogues for patients with diabetes mellitus type 2."[11] More recent randomized controlled trials have found no differences with glargine[12] and have found that although long acting insulins were less effective, they were associated with less hypoglycemia.[13]
Premixed insulins
Mixtures can be of two types of human insulins or two insulin analogues. Human mixtures and analogue mixtures have similar efficacy.[4] Mixtures of human insulin:
- A mixture of NPH and regular insulin – starts working in 30 minutes and is active 16 to 24 hours. There are several variations with different proportions of the mixed insulins.
Mixtures of analogue insulin:
- NPL/lispro 75/25
Modes of administration
Unlike many medicines, insulin cannot be taken orally. Like nearly all other proteins introduced into the gastrointestinal tract, it is reduced to fragments (even single amino acid components), whereupon all 'insulin activity' is lost.
Subcutaneous
Insulin is usually taken as subcutaneous injections by single-use syringes with needles, an insulin pump, or by repeated-use insulin pens with needles.
Insulin pump
Insulin pumps are a reasonable solution for some. Advantages to the patient are better control over background or 'basal' insulin dose, bolus doses calculated to fractions of a unit, and calculators in the pump that help with dosing 'bolus' infusions. The limitations are cost, the potential for hypoglycemic and hyperglycemic episodes, catheter problems, and no "closed loop" means of controlling insulin delivery based on current blood glucose levels.
Insulin pumps may be like 'electrical injectors' attached to a semi-permanently implanted catheter or cannula. Some who cannot achieve adequate glucose control by conventional (or jet) injection are able to do so with the appropriate pump.
As with injections, if too much insulin is delivered or the patient eats less than he or she dosed for, there will be hypoglycemia. On the other hand, if too little insulin is delivered, there will be hyperglycemia. Both can be life-threatening. In addition, indwelling catheters pose the risk of infection and ulceration. These risks can be minimized by keeping infusion sites clean. Insulin pumps require care and effort to use correctly. However, some diabetics are capable to keep their glucose in reasonable control only on a pump.
Inhalation
In 2006 the U.S. Food and Drug Administration approved the use of Exubera, the first inhalable insulin.[14] Inhaled insulin has similar efficacy to injected insulin, both in terms of controlling glucose levels and blood half-life. Currently, inhaled insulin is short acting and is typically taken before meals; an injection of long-acting insulin at night is often still required.[15] When patients were switched from injected to inhaled insulin, no significant difference was found in HbA1c levels over three months. Accurate dosing is still a problem, but patients showed no significant weight gain or pulmonary function decline over the length of the trial, when compared to the baseline.[16] Following its commercial launch in 2005 in the UK, it has not (as of July 2006) been recommended by National Institute for Health and Clinical Excellence for routine use, except in cases where there is "proven injection phobia diagnosed by a psychiatrist or psychologist".[15]
Transdermal
There are several methods for transdermal delivery of insulin. Pulsatile insulin uses microjets to pulse insulin into the patient, mimicking the physiological secretions of insulin by the pancreas.[17] Jet injection (also sometimes used for vaccinations) had different insulin delivery peaks and durations as compared to needle injection. Some diabetics find control possible with jet injectors, but not with hypodermic injection.
Both electricity using iontophoresis[18] and ultrasound have been found to make the skin temporarily porous. The insulin administration aspect remains experimental, but the blood glucose test aspect of 'wrist appliances' is commercially available.
Researchers have produced a watch-like device that tests for blood glucose levels through the skin and administers corrective doses of insulin through pores in the skin.
Intranasal insulin
Intranasal insulin is being investigated.[19]
Oral insulin
The basic appeal of oral hypoglycemic agents is that most people would prefer a pill to an injection. However, insulin is a protein. Protein hormones, like meat proteins, are digested in the stomach and gut.
The potential market for an oral form of insulin is enormous and many laboratories have attempted to devise ways of moving enough intact insulin from the gut to the portal vein to have a measurable effect on blood sugar. One can find several research reports over the years describing promising approaches or limited success in animals, and limited human testing, but as of 2004, no products appear to be successful enough to bring to market.[20]
Pancreatic transplantation
Another improvement would be a transplantation of the pancreas or beta cell to avoid periodic insulin administration. This would result in a self-regulating insulin source. Transplantation of an entire pancreas (as an individual organ) is difficult and uncommon. Generally, it is performed in conjunction with liver or kidney transplant. However, it is possible to do a transplantation of only the pancreatic beta cells. It has been highly experimental (for which read 'prone to failure') for many years, but some researchers in Alberta, Canada, have developed techniques with a high initial success rate (about 90% in one group). Nearly half of those who got an islet cell transplant are insulin-free one year after the operation; by the end of the second year that number drops to about one in seven. Beta cell transplant may become practical in the near future. Additionally, some researchers have explored the possibility of transplanting genetically engineered non-beta cells to secrete insulin.[21] Clinically testable results are far from realization. Several other non-transplant methods of automatic insulin delivery are being developed in research labs, but none is close to clinical approval.
Artificial pancreas
Adverse effects
There is conflicting evidence whether administration of exogenous insulin may increase cancer risk.[22] The increased risk may be due to increased affinity of insulin analogues to insulin-like growth factor I.[23]
| Study | Study design | Finding |
|---|---|---|
| German health insurance[24] | cohort study • 127,031 patients • mean follow-up of 1.63 years |
"the cancer incidence with glargine was higher than expected compared with human insulin" |
| Sweden prescription database[25] | cohort study • 114,841 patients • follow-up < 2.5 years |
"women using insulin glargine alone (no other types of insulin) had an increased incidence rate of breast cancer as compared with women using types of insulin other than insulin glargine." |
| UK general practices[26] | cohort study • 62,809 patients • follow-up < 9 years |
"Those on insulin or insulin secretagogues were more likely to develop solid cancers than those on metformin, and combination with metformin abolished most of this excess risk" |
| Scottish Diabetes Research Network[27] | cohort study • 36,254 patients • follow-up 4 years |
"Overall, insulin glargine use was not associated with an increased risk of all cancers or site-specific cancers in Scotland over a 4 year time frame. Given the overall data, we consider the excess of cases of all cancers and breast cancer in the subgroup of insulin glargine only users to more likely reflect allocation bias rather than an effect of insulin glargine itself" |
| North American trial[28] | randomized controlled trial • 1017 patients • follow-up 4 years |
"data reported here also confirm that there was no evidence of any difference in the rate of benign or malignant tumour development with insulin glargine compared with NPH insulin." |
In many patients, insulin is an appetite stimulant; it is sometimes used off-label specifically for that purpose. Like most diabetes treatments, other than metformin and some of the newer classes such as incretin analogues, it often causes weight gain.
References
- ↑ Coordinates determined by Norrman, Hubalek and Schluckebier (2007) Structural characterization of insulin NPH formulations. Eur.J.Pharm.Sci. 30: 414-423.
- ↑ http://www.medscape.com/viewarticle/438368
- ↑ Singh SR et al. (February 2009). "Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta-analysis". CMAJ 180: 385–97. DOI:10.1503/cmaj.081041. PMID 19221352. PMC 2638025. Research Blogging.
- ↑ 4.0 4.1 Qayyum R et al. (October 2008). "Systematic review: comparative effectiveness and safety of premixed insulin analogues in type 2 diabetes". Ann Intern Med 149: 549–59. PMID 18794553. [e]
- ↑ Majumdar S, Barrett E (October 2008). "Newer insulins in search of a niche". Annals of internal medicine 149: 586–8. PMID 18936506. [e]
- ↑ Rapid-Acting Insulin Analogs The Medical Letter
- ↑ The most up-to-date information about Insulin lispro and other drugs can be found at the following sites.
- Insulin lispro - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Insulin lispro - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Insulin lispro - Detailed information from DrugBank.
- ↑ The most up-to-date information about Insulin aspart and other drugs can be found at the following sites.
- Insulin aspart - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Insulin aspart - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Insulin aspart - Detailed information from DrugBank.
- ↑ The most up-to-date information about Insulin glargine and other drugs can be found at the following sites.
- Insulin glargine - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Insulin glargine - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Insulin glargine - Detailed information from DrugBank.
- ↑ The most up-to-date information about Insulin detemir and other drugs can be found at the following sites.
- Insulin detemir - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Insulin detemir - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Insulin detemir - Detailed information from DrugBank.
- ↑ Horvath K et al. (2007). "Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus". Cochrane database of systematic reviews (Online): CD005613. PMID 17443605.
- ↑ Esposito K et al. (2008). "Addition of neutral protamine lispro insulin or insulin glargine to oral type 2 diabetes regimens for patients with suboptimal glycemic control: a randomized trial". Ann Intern Med 149: 531–9. PMID 18936501. [e]
- ↑ Holman RR et al. (2007). "Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes". N Engl J Med 357: 1716–30. DOI:10.1056/NEJMoa075392. PMID 17890232. Research Blogging.
- ↑ FDA approval of Exubera inhaled insulin
- ↑ 15.0 15.1 NICE (June 21 2006). Diabetes (type 1 and 2), Inhaled Insulin - Appraisal Consultation Document (second). Retrieved on 2006-07-26.
- ↑ Cefalu W, Skyler J, Kourides I, Landschulz W, Balagtas C, Cheng S, Gelfand R (2001). "Inhaled human insulin treatment in patients with type 2 diabetes mellitus". Ann Intern Med 134 (3): 203–7. PMID 11177333.
- ↑ Arora A, Hakim I, Baxter J, et al (2007). "Needle-free delivery of macromolecules across the skin by nanoliter-volume pulsed microjets". Proc. Natl. Acad. Sci. U.S.A. 104 (11): 4255–60. DOI:10.1073/pnas.0700182104. PMID 17360511. Research Blogging.
- ↑ Dixit N, Bali V, Baboota S, Ahuja A, Ali J (2007). "Iontophoresis - an approach for controlled drug delivery: a review". Current drug delivery 4 (1): 1–10. PMID 17269912. [e]
- ↑ Lalej-Bennis D, Boillot J, Bardin C, et al (2001). "Efficacy and tolerance of intranasal insulin administered during 4 months in severely hyperglycaemic Type 2 diabetic patients with oral drug failure: a cross-over study". Diabet. Med. 18 (8): 614–8. PMID 11553197. [e]
- ↑ Oral Insulin - Fact or Fiction? - Resonance - May 2003. Retrieved on 2007-09-23.
- ↑ Yong Lian Zhu et al. (2003). "Aggregation and Lack of Secretion of Most Newly Synthesized Proinsulin in Non-ß-Cell Lines". Endocrinology 145 (8): 3840–3849.
- ↑ Smith U, Gale EA (September 2009). "Does diabetes therapy influence the risk of cancer?". Diabetologia 52 (9): 1699–708. DOI:10.1007/s00125-009-1441-5. PMID 19597799. Research Blogging.
- ↑ Kurtzhals P, Schäffer L, Sørensen A, Kristensen C, Jonassen I, Schmid C et al. (2000). "Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use.". Diabetes 49 (6): 999-1005. PMID 10866053.
- ↑ 24.0 24.1 Hemkens LG, Grouven U, Bender R, et al. (September 2009). "Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study". Diabetologia 52 (9): 1732–44. DOI:10.1007/s00125-009-1418-4. PMID 19565214. PMC 2723679. Research Blogging.
- ↑ 25.0 25.1 Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steineck G (September 2009). "Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden". Diabetologia 52 (9): 1745–54. DOI:10.1007/s00125-009-1444-2. PMID 19588120. Research Blogging.
- ↑ 26.0 26.1 Currie CJ, Poole CD, Gale EA (September 2009). "The influence of glucose-lowering therapies on cancer risk in type 2 diabetes". Diabetologia 52 (9): 1766–77. DOI:10.1007/s00125-009-1440-6. PMID 19572116. Research Blogging.
- ↑ 27.0 27.1 Colhoun HM (September 2009). "Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group". Diabetologia 52 (9): 1755–65. DOI:10.1007/s00125-009-1453-1. PMID 19603149. PMC 2723678. Research Blogging.
- ↑ 28.0 28.1 Rosenstock J, Fonseca V, McGill JB, et al. (September 2009). "Similar risk of malignancy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: findings from a 5 year randomised, open-label study". Diabetologia 52 (9): 1971–3. DOI:10.1007/s00125-009-1452-2. PMID 19609501. PMC 2723677. Research Blogging.