Nitrogen cycle: Difference between revisions
imported>Milton Beychok m (→Different parts of the nitrogen cycle: Put a small buffer around the table ... the text was too close.) |
mNo edit summary |
||
| (14 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{Image|Nitrogen Cycle drawing.png|right|420px|The nitrogen cycle is a complex system necessary for living beings.}} | |||
{{TOC|right}} | {{TOC|right}} | ||
The '''nitrogen cycle''', more specifically called the '''biogeochemical nitrogen cycle''',<ref name=nasa1>[http://soil.gsfc.nasa.gov/NFTG/nitrocyc.htm The Nitrogen Cycle: Nitrogen Transformations in Soil, Water, and Air.] Soil Education Project. National Aeronautics and Space Agency (NASA)</ref> — a life-sustaining nutrient cycle of Earth's [[biosphere]] — operates as a multi-stage process involving the movement of atmospheric [[nitrogen]] | The '''nitrogen cycle''', more specifically called the '''biogeochemical nitrogen cycle''',<ref name=nasa1>[http://soil.gsfc.nasa.gov/NFTG/nitrocyc.htm The Nitrogen Cycle: Nitrogen Transformations in Soil, Water, and Air.] Soil Education Project. National Aeronautics and Space Agency (NASA)</ref> | ||
</ref> into the Earth's soil and water compartments through rainfall, and, for most of them, back again into the atmosphere after soil- and water-bound nitrogen atoms have passed through many different organic and inorganic compounds by many different chemical processes. The biogeochemical cycling of nitrogen atoms serves to sustain living systems, nitrogen contributing one component of many of life's essential molecules, such as [[amino acids]], [[proteins]], and [[nucleic acids]] like [[DNA]] and [[RNA]], and a necessary element for [[Photosynthesis|photosynthetic organisms]], upon which nearly all Earth's living systems depend.<ref name=twsMAR26b>{{cite news | — a life-sustaining nutrient cycle of Earth's [[biosphere]] — operates as a multi-stage process involving the movement of atmospheric [[nitrogen]] molecules (N<sub>2</sub>), the biosphere's main site and form of nitrogen, and small amounts of other atmospheric nitrogen-containing [[ion]]s and compounds, for example [[nitrate anion]]s (NO<sub>3</sub><sup>−</sup>), [[nitric oxide]] (NO), [[nitrogen dioxide]] (NO<sub>2</sub>), [[nitrous oxide]] (N<sub>2</sub>O), [[ammonium cation]]s (NH<sub>4</sub><sup>+</sup>),<ref name=johnson2003>Harrison, J.A. (2003) [http://visionlearning.com/library/module_viewer.php?mid=98&l=&c3= The Nitrogen Cycle: Of Microbes and Men.] ''VisionLearning'' EAS-2(4).</ref> | ||
into the Earth's soil and water compartments through rainfall, and, for most of them, back again into the atmosphere after soil- and water-bound nitrogen atoms have passed through many different organic and inorganic compounds by many different chemical processes. The biogeochemical cycling of nitrogen atoms serves to sustain living systems, nitrogen contributing one component of many of life's essential molecules, such as [[amino acids]], [[proteins]], and [[nucleic acids]] like [[DNA]] and [[RNA]], and a necessary element for [[Photosynthesis|photosynthetic organisms]], upon which nearly all Earth's living systems depend.<ref name=twsMAR26b>{{cite news | |||
|title= The Nitrogen Cycle | |title= The Nitrogen Cycle | ||
|publisher= Kimball | |publisher= Kimball | ||
|quote= All life requires nitrogen-compounds, e.g., proteins and nucleic acids.* Air, which is 79% nitrogen gas ( | |quote= All life requires nitrogen-compounds, e.g., proteins and nucleic acids.* Air, which is 79% nitrogen gas (N<sub>2</sub>), is the major reservoir of nitrogen.* But most organisms cannot use nitrogen in this form.* Plants must secure their nitrogen in "fixed" form, i.e., incorporated in compounds such as: nitrate ions (NO<sub>3</sub><sup>−</sup>) ammonia (NH<sub>3</sub>) urea (NH<sub>2</sub>)<sub>2</sub>CO * Animals secure their nitrogen (and all other) compounds from plants (or animals that have fed on plants). | ||
|date= 2010-03-26 | |date= 2010-03-26 | ||
|url= http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/N/NitrogenCycle.html | |url= http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/N/NitrogenCycle.html | ||
|accessdate= 2010-03-26 | |||
}}</ref> <ref name=twsMAR26a>{{cite news | |||
|title= The Nitrogen Cycle: Nitrogen Transformations in Soil, Water, and Air | |||
|publisher= Soil Science Education Home Page (via NASA) | |||
|date= 2004 | |||
|url= http://soil.gsfc.nasa.gov/NFTG/nitrocyc.htm | |||
|accessdate= 2010-03-26 | |accessdate= 2010-03-26 | ||
}}</ref> | }}</ref> | ||
While nitrogen is abundant in [[air]] as di-nitrogen molecules (N<sub>2</sub>) (~10<sup>6</sup> times greater than all the nitrogen in Earth's organisms)<ref name=pgnetncycle>[http://www.physicalgeography.net/fundamentals/9s.html The Nitrogen Cycle.] Chapter 9: Introduction to the Biosphere. PhysicalGeography.net Fundamentals eBook.</ref> and prevents oxygen from [[combustion]], the air-born nitrogen is generally not useful to living beings; it must be transformed to other compounds to enable its use by living systems. | While nitrogen is abundant in [[air]] as di-nitrogen molecules (N<sub>2</sub>) (in amounts ~10<sup>6</sup> times greater than all the nitrogen in Earth's organisms)<ref name=pgnetncycle>[http://www.physicalgeography.net/fundamentals/9s.html The Nitrogen Cycle.] Chapter 9: Introduction to the Biosphere. PhysicalGeography.net Fundamentals eBook.</ref> and prevents oxygen from [[combustion]], the air-born nitrogen is generally not useful to living beings; it must be transformed to other compounds to enable its use by living systems. Di-nitrogen has a triple chemical covalent bond, and therefore is relatively [[inert]], requiring considerable [[energy]] to render it biologically available.<ref name=johnson2003/> <ref name=twsMAR26c>{{cite news | ||
|title= CHM 110 - CHEMISTRY AND ISSUES IN THE ENVIRONMENT | |title= CHM 110 - CHEMISTRY AND ISSUES IN THE ENVIRONMENT | ||
|publisher= Elmhurst | |publisher= Elmhurst | ||
| Line 19: | Line 27: | ||
|url= http://www.elmhurst.edu/~chm/onlcourse/chm110/outlines/nitrogencycle.html | |url= http://www.elmhurst.edu/~chm/onlcourse/chm110/outlines/nitrogencycle.html | ||
|accessdate= 2010-03-26 | |accessdate= 2010-03-26 | ||
}}</ref> | }}</ref> That occurs naturally, by the action of microorganisms, and by human activity. | ||
Nitrogen is an important element in the production of [[food]], as all organisms require it to maintain their living state, plants mostly as nitrate anion (NO<sub>3</sub><sup>−</sup>) and to some extent as ammonium cation (NH<sub>4</sub><sup>+</sup>), animals as nitrogen-containing organic compounds from living or dead organic matter. | Nitrogen is an important element in the production of [[food]], as all organisms require it to maintain their living state, plants mostly as nitrate anion (NO<sub>3</sub><sup>−</sup>) and to some extent as ammonium cation (NH<sub>4</sub><sup>+</sup>), animals as nitrogen-containing organic compounds from living or dead organic matter. | ||
| Line 37: | Line 45: | ||
* [[Urea]] ((NH<sub>2</sub>)<sub>2</sub>CO) | * [[Urea]] ((NH<sub>2</sub>)<sub>2</sub>CO) | ||
* Nitrate anions (NO<sub>3</sub><sup>−</sup>)<ref name=twsMAR26b/> | * Nitrate anions (NO<sub>3</sub><sup>−</sup>)<ref name=twsMAR26b/> | ||
* [[Nitrite]] anions (NO<sub>2</sub><sup>−</sup>)<ref name=twsMAR26a/><ref name=twsMAR26b> | * [[Nitrite]] anions (NO<sub>2</sub><sup>−</sup>)<ref name=twsMAR26a/><ref name=twsMAR26b/> | ||
Animals get their necessary nitrogen from eating plants. Getting nitrogen out of the atmosphere is key for food production. In 1908, [[Germany|German]] scientist [[Fritz Haber]] pioneered a way to get nitrogen out of the atmosphere to create a better fertilizer, and the innovation was called the Haber-Bosch process. It produces liquid ammonia which is the raw material for nitrogen fertilizer. Today fertilizer factories pour out 100 million tons of nitrogen annually, allowing an estimated two billion more people have food. But this radical change has been a cause for concern.<ref name=twsMAR26d>{{cite news | Animals get their necessary nitrogen from eating plants. Getting nitrogen out of the atmosphere is key for food production. In 1908, [[Germany|German]] scientist [[Fritz Haber]] pioneered a way to get nitrogen out of the atmosphere to create a better fertilizer, and the innovation was called the Haber-Bosch process. It produces liquid ammonia which is the raw material for nitrogen fertilizer. Today fertilizer factories pour out 100 million tons of nitrogen annually, allowing an estimated two billion more people have food. But this radical change has been a cause for concern.<ref name=twsMAR26d>{{cite news | ||
|author= Dan Charles | |author= Dan Charles | ||
| Line 48: | Line 56: | ||
}}</ref> | }}</ref> | ||
Some nitrates and nitrites can leach out into the soil into the [[groundwater]] and change into nitrogen gases such as | Some nitrates and nitrites can leach out into the soil into the [[groundwater]] and change into nitrogen gases such as N<sub>2</sub>, NO, N<sub>2</sub>0, and ammonia gas (NH<sub>3</sub>). When they escape out of the soil, it is called "volatizing". It goes into the [[atmosphere]] of the [[Earth (planet)|Earth]].<ref name=twsMAR26a/> | ||
==Different parts of the nitrogen cycle== | ==Different parts of the nitrogen cycle== | ||
{{Image|Lightning over Oradea Romania 3.jpg|right|260px|The intense energy of a [[lightning]] strike can ''fix'' nitrogen, but the overall amount of nitrogen fixation by lightning strikes is small compared with the action of bacteria.}} | |||
{{Image|Nitrogen Cycle by T Sulcer.jpg|right|420px|Organic nitrogen is useless to plants, but must be converted into inorganic form to once again be useful.}} | {{Image|Nitrogen Cycle by T Sulcer.jpg|right|420px|Organic nitrogen is useless to plants, but must be converted into inorganic form to once again be useful.}} | ||
These include: | These include: | ||
* [[Ammonification]] is when nitrogen in organic form is converted by [[microorganisms]] into ammonium cations (NH<sub>4</sub><sup>+</sup>). It has a positive charge and this lets it become [[adsorption|adsorbed]] and [[fixation (chemistry)|fixated]] to the negatively charged soil particles, or be taken in by plants. When a plant or animal dies or makes waste, the nitrogen is organic and must be changed by either bacteria or [[fungi]] into ammonium (NH<sub>4</sub><sup>+</sup>), a process called ammonification or mineralization. Some microorganisms are not properly called bacteria but are called [[archaea]], which have a genetic makeup similar to humans, and are classified as [[eukaryotes]], and they sometimes have the ability to live in "extreme" environments such as inside the mouths of active volcanoes, or in extreme cold such as [[Antarctica]], or in very acidic or very saline environments (e.g., the [[Dead Sea]].) | * [[Ammonification]] is when nitrogen in organic form is converted by [[microorganisms]] into ammonium cations (NH<sub>4</sub><sup>+</sup>). It has a positive charge and this lets it become [[adsorption|adsorbed]] and [[fixation (chemistry)|fixated]] to the negatively charged soil particles, or be taken in by plants. When a plant or animal dies or makes waste, the nitrogen is organic and must be changed by either bacteria or [[Fungus|fungi]] into ammonium (NH<sub>4</sub><sup>+</sup>), a process called ammonification or mineralization. Some microorganisms are not properly called bacteria but are called [[archaea]], which have a genetic makeup similar to humans, and are classified as [[eukaryotes]], and they sometimes have the ability to live in "extreme" environments such as inside the mouths of active volcanoes, or in extreme cold such as [[Antarctica]], or in very acidic or very saline environments (e.g., the [[Dead Sea]].) | ||
* [[Decay]] is a process of transforming excrement which contains organic nitrogen by microorganisms; the molecules are broken down, so that excretions and dead organisms turn into ammonia. | * [[Decay]] is a process of transforming excrement which contains organic nitrogen by microorganisms; the molecules are broken down, so that excretions and dead organisms turn into ammonia. | ||
| Line 67: | Line 75: | ||
::#[[Lightning]] strikes, which have enormous energy to break down inert nitrogen molecules, allowing them to combine with oxygen making nitrogen oxides; these can dissolve in rain, forming "nitrates", that are carried by gravity to the earth. | ::#[[Lightning]] strikes, which have enormous energy to break down inert nitrogen molecules, allowing them to combine with oxygen making nitrogen oxides; these can dissolve in rain, forming "nitrates", that are carried by gravity to the earth. | ||
::#[[Symbiotic bacteria]] which have the nitrogenase [[enzyme]] that combines gaseous nitrogen with [[hydrogen]] to produce [[ammonia]], which is then further converted by the bacteria to make their organic compounds needed by the bacterium for internal processes. Some nitrogen fixing bacteria, such as [[Rhizobium]], live in the root nodules of [[legumes]] and work with the plant as a form of [[symbiosis]] and produce [[ammonia]] in exchange for [[carbohydrates]]. If a soil is lacking valuable nutrients, it's possible to plant legumes to add the nitrogen quotient.<ref name=twsMAR26b/> It's possible for nitrogen fixing bacteria to take nitrogen from the air and convert it into ammonia (NH<sub>3</sub>). It goes into a variety of amino acids and helps make proteins.<ref name=twsMAR26c/> | ::#[[Symbiotic bacteria]] which have the nitrogenase [[enzyme]] that combines gaseous nitrogen with [[hydrogen]] to produce [[ammonia]], which is then further converted by the bacteria to make their organic compounds needed by the bacterium for internal processes. Some nitrogen fixing bacteria, such as [[Rhizobium]], live in the root nodules of [[legumes]] and work with the plant as a form of [[symbiosis]] and produce [[ammonia]] in exchange for [[carbohydrates]]. If a soil is lacking valuable nutrients, it's possible to plant legumes to add the nitrogen quotient.<ref name=twsMAR26b/> It's possible for nitrogen fixing bacteria to take nitrogen from the air and convert it into ammonia (NH<sub>3</sub>). It goes into a variety of amino acids and helps make proteins.<ref name=twsMAR26c/> | ||
::#[[Industrial fixation]] is a third way. Under great pressure at 600°C, and using a [[catalyst]], atmospheric nitrogen and hydrogen (usually derived from [[natural gas]] or [[petroleum]]) can be combined to form ammonia ( | ::#[[Industrial fixation]] is a third way. Under great pressure at 600°C, and using a [[catalyst]], atmospheric nitrogen and hydrogen (usually derived from [[natural gas]] or [[petroleum]]) can be combined to form ammonia (NH<sub>3</sub>) which can be used directly as fertilizer, but for economic reasons, mostly it's processed further to yield urea and ammonium nitrate (NH<sub>4</sub>NO<sub>3</sub>).<ref name=twsMAR26b/> | ||
* [[Haber process|Haber Process]] is a synthetic process in which nitrogen and hydrogen react under great pressure and temperature in the presence of a [[Catalysis|catalyst]] to make ammonia.<ref name=twsMAR26c/> It was named after [[Fritz Haber]], a [[Nobel]] laureate who helped transform food production worldwide. | * [[Haber process|Haber Process]] is a synthetic process in which nitrogen and hydrogen react under great pressure and temperature in the presence of a [[Catalysis|catalyst]] to make ammonia.<ref name=twsMAR26c/> It was named after [[Fritz Haber]], a [[Nobel]] laureate who helped transform food production worldwide. | ||
| Line 131: | Line 139: | ||
* [[Mineralization]] can happen to nitrogen, like it can happen to any element, when it's converted from an organic to an inorganic form by microbes, and can be used by plants for growing. | * [[Mineralization]] can happen to nitrogen, like it can happen to any element, when it's converted from an organic to an inorganic form by microbes, and can be used by plants for growing. | ||
* [[Nitrification]] happens under certain conditions. Microbes in the soil use [[ammonium nitrate]] in the soil for energy and therefore [[oxidation|oxidize]] ammonium nitrogen first into Nitrite Nitrogen (NO2-) and then into Nitrate Nitrogen ( | * [[Nitrification]] happens under certain conditions. Microbes in the soil use [[ammonium nitrate]] in the soil for energy and therefore [[oxidation|oxidize]] ammonium nitrogen first into Nitrite Nitrogen (NO2-) and then into Nitrate Nitrogen (NO<sub>3</sub><sup>-</sup>). It's primarily done by bacteria in the soil such as [[Nitrosomonas]]. It's very important for nitrites to be converted to nitrates because the former are toxic to plants; if nitrites keep building up, it will eventually harm plant life. Because nitrates are highly soluble, it's easy for them to enter the groundwater, but it can affect drinking water; it's more dangerous for young human babies because it can interfere with their blood-oxygen levels and cause a condition known as [[methemoglobinemia]]. Also, when there is too much nitrates in streams, it can lead to excessive growth of [[algae]] which can lead to the death of aquatic life such as fishes because of the algae's excessive demand for oxygen. | ||
* [[Organic nitrogen]] can't be used by plants. But it's formed when living matter dies, such as plant roots, leaves, sticks, animals including humans and insects. It's also formed when animals excrete and make manure, compost, and sewage sludge. The process of [[decomposition]] is when organic nitrogen is broken down into inorganic nitrogen by microbes. | * [[Organic nitrogen]] can't be used by plants. But it's formed when living matter dies, such as plant roots, leaves, sticks, animals including humans and insects. It's also formed when animals excrete and make manure, compost, and sewage sludge. The process of [[decomposition]] is when organic nitrogen is broken down into inorganic nitrogen by microbes. | ||
| Line 142: | Line 150: | ||
==Impact of humans== | ==Impact of humans== | ||
There is concern that a number of activities by humans have influenced the nitrogen cycle, and it is not clear what ramifications these changes have had or will continue to have. Of interest is the growing of certain types of plants such as [[soy]],<ref name=twsMAR26b/> [[alfalfa]]<ref name=twsMAR26b/> and [[clover]], the use of chemical fertilizers in soil to enhance crop yields, and pollution emitted by cars and trucks and planes and industrial activity. There is speculation that | There is concern that a number of activities by humans have influenced the nitrogen cycle, and it is not clear what ramifications these changes have had or will continue to have. Of interest is the growing of certain types of plants such as [[soy]],<ref name=twsMAR26b/> [[alfalfa]]<ref name=twsMAR26b/> and [[clover]], the use of chemical fertilizers in soil to enhance crop yields, and pollution emitted by cars and trucks and planes and industrial activity. There is speculation that N<sub>2</sub>O, or [[nitrous oxide]] can have negative affects in the atmosphere; this is believed to be exacerbated by agricultural use of fertilizers, the burning of biomass and cattle and feedlots, and industrial output. There is concern that nitrous oxide can have a negative affect on the atmospheric layer known as [[ozone]], since the ozone layer is believed to shield the earth from dangerous rays from the [[sun]]. Further, changes in the cycle may have resulted in more ammonia in the air which can decrease air quality and cling to water droplets, possibly resulting in [[acid rain]]. Last, there is concern that changes in the cycle have led to contamination by certain areas of nitrates, with speculation about the safety of drinking water, that is, they pollute drinking water.<ref name=twsMAR26d/> | ||
There is a movement among [[biogeochemists]] to see the nitrogen cycle as it relates to the cycles of other compounds in the earth, such as the [[water cycle]], or the cycling of other chemical compounds.<ref name=twsMAR26e>{{cite news | There is a movement among [[biogeochemists]] to see the nitrogen cycle as it relates to the cycles of other compounds in the earth, such as the [[water cycle]], or the cycling of other chemical compounds.<ref name=twsMAR26e>{{cite news | ||
| Line 152: | Line 160: | ||
}}</ref> A reporter explained: | }}</ref> A reporter explained: | ||
<blockquote>A biogeochemical cycle is a pathway by which a chemical element, such as carbon, or compound, like water, moves through Earth's biosphere, atmosphere, hydrosphere and lithosphere. In effect, the element is "recycled," although in some cycles the element is accumulated or held for long periods of time. Chemical compounds are passed from one organism to another, and from one part of the biosphere to another, through biogeochemical cycles. Water, for example, can go through three phases (liquid, solid, gas) as it cycles through the Earth system. It evaporates from plants as well as land and ocean surfaces into the atmosphere and, after condensing in clouds, returns to Earth as rain and snow. Researchers are discovering that biogeochemical cycles--whether the water cycle, the nitrogen cycle, the [[carbon cycle]], or others--happen in concert with one another. Biogeochemical cycles are "coupled" to each other and to Earth's physical features.<ref name=twsMAR26e/></blockquote> | <blockquote>A biogeochemical cycle is a pathway by which a chemical element, such as carbon, or compound, like water, moves through Earth's biosphere, atmosphere, hydrosphere and lithosphere. In effect, the element is "recycled," although in some cycles the element is accumulated or held for long periods of time. Chemical compounds are passed from one organism to another, and from one part of the biosphere to another, through biogeochemical cycles. Water, for example, can go through three phases (liquid, solid, gas) as it cycles through the Earth system. It evaporates from plants as well as land and ocean surfaces into the atmosphere and, after condensing in clouds, returns to Earth as rain and snow. Researchers are discovering that biogeochemical cycles--whether the water cycle, the nitrogen cycle, the [[carbon cycle]], or others--happen in concert with one another. Biogeochemical cycles are "coupled" to each other and to Earth's physical features.<ref name=twsMAR26e/></blockquote> | ||
==References== | ==References== | ||
{{reflist}} | {{reflist}}[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 07:01, 26 September 2024
The nitrogen cycle, more specifically called the biogeochemical nitrogen cycle,[1] — a life-sustaining nutrient cycle of Earth's biosphere — operates as a multi-stage process involving the movement of atmospheric nitrogen molecules (N2), the biosphere's main site and form of nitrogen, and small amounts of other atmospheric nitrogen-containing ions and compounds, for example nitrate anions (NO3−), nitric oxide (NO), nitrogen dioxide (NO2), nitrous oxide (N2O), ammonium cations (NH4+),[2] into the Earth's soil and water compartments through rainfall, and, for most of them, back again into the atmosphere after soil- and water-bound nitrogen atoms have passed through many different organic and inorganic compounds by many different chemical processes. The biogeochemical cycling of nitrogen atoms serves to sustain living systems, nitrogen contributing one component of many of life's essential molecules, such as amino acids, proteins, and nucleic acids like DNA and RNA, and a necessary element for photosynthetic organisms, upon which nearly all Earth's living systems depend.[3] [4]
While nitrogen is abundant in air as di-nitrogen molecules (N2) (in amounts ~106 times greater than all the nitrogen in Earth's organisms)[5] and prevents oxygen from combustion, the air-born nitrogen is generally not useful to living beings; it must be transformed to other compounds to enable its use by living systems. Di-nitrogen has a triple chemical covalent bond, and therefore is relatively inert, requiring considerable energy to render it biologically available.[2] [6] That occurs naturally, by the action of microorganisms, and by human activity.
Nitrogen is an important element in the production of food, as all organisms require it to maintain their living state, plants mostly as nitrate anion (NO3−) and to some extent as ammonium cation (NH4+), animals as nitrogen-containing organic compounds from living or dead organic matter.
Thus, on Earth, atoms of the chemical element nitrogen (symbol, N), an essential element for Earth's living systems — undergo cyclical movement through the atmosphere, the crust (lithosphere), water compartments (hydrosphere), living systems, and non-living organic matter, a multi-stage processes effected by natural reactions, but influenced significantly as well by man-made activity (see below).[4]
When animals defecate or when plants decompose, they produce manure, sewage waste, compost, rotting leaves and sticks and other matter, and the result is waste that contains organic nitrogen. The result is organic soil material called humus. In addition, inorganic nitrogen comes from different sources as well; for example, certain minerals have nitrogen, as well as nitrogen coming from precipitation such as rain or snow, and fertilizers have nitrogen as well. This added nitrogen in the soil makes it easier for living plants to grow healthy. While plants can't use some types of organic nitrogen, it's necessary for microbes living in the soil to convert the organic nitrogen into inorganic nitrogen which enables the plants to use it for their benefit.[4]
Plants use a variety of inorganic or "fixed" nitrogen, which means that they have their nitrogen incorporated into compounds such as:
- Soil-based inorganic nitrogen in the form of ammonium cations (NH4+)
- Urea ((NH2)2CO)
- Nitrate anions (NO3−)[3]
- Nitrite anions (NO2−)[4][3]
Animals get their necessary nitrogen from eating plants. Getting nitrogen out of the atmosphere is key for food production. In 1908, German scientist Fritz Haber pioneered a way to get nitrogen out of the atmosphere to create a better fertilizer, and the innovation was called the Haber-Bosch process. It produces liquid ammonia which is the raw material for nitrogen fertilizer. Today fertilizer factories pour out 100 million tons of nitrogen annually, allowing an estimated two billion more people have food. But this radical change has been a cause for concern.[7]
Some nitrates and nitrites can leach out into the soil into the groundwater and change into nitrogen gases such as N2, NO, N20, and ammonia gas (NH3). When they escape out of the soil, it is called "volatizing". It goes into the atmosphere of the Earth.[4]
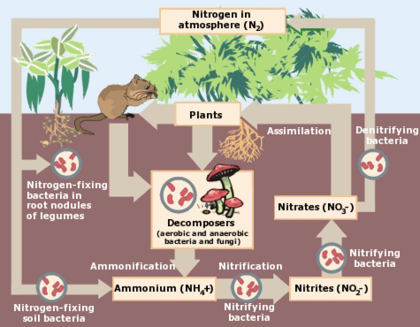
Different parts of the nitrogen cycle

The intense energy of a lightning strike can fix nitrogen, but the overall amount of nitrogen fixation by lightning strikes is small compared with the action of bacteria.
These include:
- Ammonification is when nitrogen in organic form is converted by microorganisms into ammonium cations (NH4+). It has a positive charge and this lets it become adsorbed and fixated to the negatively charged soil particles, or be taken in by plants. When a plant or animal dies or makes waste, the nitrogen is organic and must be changed by either bacteria or fungi into ammonium (NH4+), a process called ammonification or mineralization. Some microorganisms are not properly called bacteria but are called archaea, which have a genetic makeup similar to humans, and are classified as eukaryotes, and they sometimes have the ability to live in "extreme" environments such as inside the mouths of active volcanoes, or in extreme cold such as Antarctica, or in very acidic or very saline environments (e.g., the Dead Sea.)
- Decay is a process of transforming excrement which contains organic nitrogen by microorganisms; the molecules are broken down, so that excretions and dead organisms turn into ammonia.
- Denitrification is when microbes transform nitrogen into nitrous oxide and nitrogen oxide gases. For this to happen, it requires soils to be extremely wet, or poorly drained. This is why wetlands are valuable since they contribute an important role in the nitrogen cycle. The resulting gases volatilize into the atmosphere, but then they're no longer available for use by plants. Denitrification involves the reduction of nitrates back into the largely inert nitrogen gas (N2), completing the nitrogen cycle. This process is performed by bacteria like Pseudomonas and Clostridium in anaerobic conditions.
- Erosion is when the soil is worn away by running water, wind, ice, gravity, human activity such as bulldozers, or by activities such as earthquakes or tsunamis or tornadoes or other geological processes. When the soil is moved by these forces, nitrogen in both organic and inorganic form in the soil are carried along.
- Fixation, sometimes better known as nitrogen fixation, is when atmospheric nitrogen is changed or fixed to make it useful by plants. It can happen by:
- Lightning strikes, which have enormous energy to break down inert nitrogen molecules, allowing them to combine with oxygen making nitrogen oxides; these can dissolve in rain, forming "nitrates", that are carried by gravity to the earth.
- Symbiotic bacteria which have the nitrogenase enzyme that combines gaseous nitrogen with hydrogen to produce ammonia, which is then further converted by the bacteria to make their organic compounds needed by the bacterium for internal processes. Some nitrogen fixing bacteria, such as Rhizobium, live in the root nodules of legumes and work with the plant as a form of symbiosis and produce ammonia in exchange for carbohydrates. If a soil is lacking valuable nutrients, it's possible to plant legumes to add the nitrogen quotient.[3] It's possible for nitrogen fixing bacteria to take nitrogen from the air and convert it into ammonia (NH3). It goes into a variety of amino acids and helps make proteins.[6]
- Industrial fixation is a third way. Under great pressure at 600°C, and using a catalyst, atmospheric nitrogen and hydrogen (usually derived from natural gas or petroleum) can be combined to form ammonia (NH3) which can be used directly as fertilizer, but for economic reasons, mostly it's processed further to yield urea and ammonium nitrate (NH4NO3).[3]
- Haber Process is a synthetic process in which nitrogen and hydrogen react under great pressure and temperature in the presence of a catalyst to make ammonia.[6] It was named after Fritz Haber, a Nobel laureate who helped transform food production worldwide.
- Immobilization is when inorganic forms of nitrogen are converted into organic forms as microbes, and when plants die. It's the opposite of mineralization. When nitrogen enters the soil in mineral form -- that is when it's not from a plant or animal source -- it's inorganic.
- Inorganic nitrogen is added to the soil by rain or snowfall or as fertilizers. This is why it's better to have actual rain water be the water source for indoor plants, since they get a generous helping of nitrogen as a result; using tap water usually doesn't bring this added benefit. In the soil, microorganisms convert organic nitrogen into inorganic forms, and this process is known as biological nitrogen fixation. Afterwards, the inorganic nitrogen is ready to be used by plants.
|
- Leaching is when nitrogen, when it's in the form of nitrate, is negatively charged. As a result, it's not attracted to the negatively charged clay and humus in the soil, that is, it's repelled, and the nitrogen won't be absorbed by the clay. Rather, it moves down through the soil into the groundwater where streams and drinking water can become contaminated. Ammonia is highly toxic to fish and, as a result, it's important to monitor the amount of ammonia in sewage treatment. One way to prevent this is to conduct nitrification before discharging materials downstream.
- Mineralization can happen to nitrogen, like it can happen to any element, when it's converted from an organic to an inorganic form by microbes, and can be used by plants for growing.
- Nitrification happens under certain conditions. Microbes in the soil use ammonium nitrate in the soil for energy and therefore oxidize ammonium nitrogen first into Nitrite Nitrogen (NO2-) and then into Nitrate Nitrogen (NO3-). It's primarily done by bacteria in the soil such as Nitrosomonas. It's very important for nitrites to be converted to nitrates because the former are toxic to plants; if nitrites keep building up, it will eventually harm plant life. Because nitrates are highly soluble, it's easy for them to enter the groundwater, but it can affect drinking water; it's more dangerous for young human babies because it can interfere with their blood-oxygen levels and cause a condition known as methemoglobinemia. Also, when there is too much nitrates in streams, it can lead to excessive growth of algae which can lead to the death of aquatic life such as fishes because of the algae's excessive demand for oxygen.
- Organic nitrogen can't be used by plants. But it's formed when living matter dies, such as plant roots, leaves, sticks, animals including humans and insects. It's also formed when animals excrete and make manure, compost, and sewage sludge. The process of decomposition is when organic nitrogen is broken down into inorganic nitrogen by microbes.
- Runoff happens when soil becomes so wet that it can no longer hold water; the water runs off the surface of the soil. If the soil has a protective layer of grasses or trees with strong roots, then the runoff will be mostly water; but if there isn't a protective covering, the runoff can erode substantial amounts of soil. This process can take both forms of nitrogen along with it -- both inorganic and organic. Sometimes this causes pollution and ends up in streams, lakes, rivers, reservoirs, and bays, and it can cause harm to aquatic life such as fishes.
- Uptake is the opposite of runoff. It's sometimes called assimilation. It happens when plant roots absorb inorganic nitrogen and other ingredients in the soil, and this helps the plants grow. Some plants get nitrogen from the soil, and by absorption of their roots in the form of either nitrate ions or ammonium ions. When animals eat plants, they get nitrogen via this pathway. Some plants can absorb nitrate or ammonium ions from the soil via their root hairs.
- Volatilization is when nitrogen is moved out of the soil and into the Earth's atmosphere in the form of a gases such as NH3, NO, N2O, or N2.[4]
Impact of humans
There is concern that a number of activities by humans have influenced the nitrogen cycle, and it is not clear what ramifications these changes have had or will continue to have. Of interest is the growing of certain types of plants such as soy,[3] alfalfa[3] and clover, the use of chemical fertilizers in soil to enhance crop yields, and pollution emitted by cars and trucks and planes and industrial activity. There is speculation that N2O, or nitrous oxide can have negative affects in the atmosphere; this is believed to be exacerbated by agricultural use of fertilizers, the burning of biomass and cattle and feedlots, and industrial output. There is concern that nitrous oxide can have a negative affect on the atmospheric layer known as ozone, since the ozone layer is believed to shield the earth from dangerous rays from the sun. Further, changes in the cycle may have resulted in more ammonia in the air which can decrease air quality and cling to water droplets, possibly resulting in acid rain. Last, there is concern that changes in the cycle have led to contamination by certain areas of nitrates, with speculation about the safety of drinking water, that is, they pollute drinking water.[7]
There is a movement among biogeochemists to see the nitrogen cycle as it relates to the cycles of other compounds in the earth, such as the water cycle, or the cycling of other chemical compounds.[8] A reporter explained:
A biogeochemical cycle is a pathway by which a chemical element, such as carbon, or compound, like water, moves through Earth's biosphere, atmosphere, hydrosphere and lithosphere. In effect, the element is "recycled," although in some cycles the element is accumulated or held for long periods of time. Chemical compounds are passed from one organism to another, and from one part of the biosphere to another, through biogeochemical cycles. Water, for example, can go through three phases (liquid, solid, gas) as it cycles through the Earth system. It evaporates from plants as well as land and ocean surfaces into the atmosphere and, after condensing in clouds, returns to Earth as rain and snow. Researchers are discovering that biogeochemical cycles--whether the water cycle, the nitrogen cycle, the carbon cycle, or others--happen in concert with one another. Biogeochemical cycles are "coupled" to each other and to Earth's physical features.[8]
References
- ↑ The Nitrogen Cycle: Nitrogen Transformations in Soil, Water, and Air. Soil Education Project. National Aeronautics and Space Agency (NASA)
- ↑ 2.0 2.1 Harrison, J.A. (2003) The Nitrogen Cycle: Of Microbes and Men. VisionLearning EAS-2(4).
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 The Nitrogen Cycle, Kimball, 2010-03-26. Retrieved on 2010-03-26. “All life requires nitrogen-compounds, e.g., proteins and nucleic acids.* Air, which is 79% nitrogen gas (N2), is the major reservoir of nitrogen.* But most organisms cannot use nitrogen in this form.* Plants must secure their nitrogen in "fixed" form, i.e., incorporated in compounds such as: nitrate ions (NO3−) ammonia (NH3) urea (NH2)2CO * Animals secure their nitrogen (and all other) compounds from plants (or animals that have fed on plants).”
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 The Nitrogen Cycle: Nitrogen Transformations in Soil, Water, and Air, Soil Science Education Home Page (via NASA), 2004. Retrieved on 2010-03-26.
- ↑ The Nitrogen Cycle. Chapter 9: Introduction to the Biosphere. PhysicalGeography.net Fundamentals eBook.
- ↑ 6.0 6.1 6.2 CHM 110 - CHEMISTRY AND ISSUES IN THE ENVIRONMENT, Elmhurst, 2010-03-26. Retrieved on 2010-03-26. “Nitrogen will only react with oxygen in the presence of high temperatures and pressures found near lightning bolts and in combustion reactions in power plants or internal combustion engines. Nitric oxide, NO, and nitrogen dioxide, NO2, are formed under these conditions. Eventually nitrogen dioxide may react with water in rain to form nitric acid, HNO3. The nitrates thus formed may be utilized by plants as a nutrient.”
- ↑ 7.0 7.1 Dan Charles. The Tragedy of Fritz Haber: Nobel Laureate Transformed World Food Production, War, NPR, July 11, 2002. Retrieved on 2010-03-26. “A young, high-strung German chemist named Fritz Haber rose to the challenge. Around 1908, he discovered a way to tap into the atmosphere's vast reservoir of nitrogen gas and convert it into compounds plants can use. The innovation, called the Haber-Bosch process, produces liquid ammonia, the raw material for making nitrogen fertilizer.”
- ↑ 8.0 8.1 Earth's Cycles, Once in Concert, Falling Out of Sync, US News, August 5, 2009. Retrieved on 2010-03-26.