Refineries: Difference between revisions
imported>Milton Beychok (Revised a wiki link) |
mNo edit summary |
||
| (4 intermediate revisions by 3 users not shown) | |||
| Line 10: | Line 10: | ||
* [[Petroleum refinery]]: Converts petroleum [[crude oil]] into [[Octane rating|high-octane]] motor fuel ([[gasoline]]/[[petrol]]), [[diesel oil]], [[liquefied petroleum gas]]es (LPG), jet aircraft fuel, [[naphtha]], [[kerosene]], heating [[fuel oil]]s, [[lubricating oil]]s, [[asphalt]] and [[petroleum coke]]. | * [[Petroleum refinery]]: Converts petroleum [[crude oil]] into [[Octane rating|high-octane]] motor fuel ([[gasoline]]/[[petrol]]), [[diesel oil]], [[liquefied petroleum gas]]es (LPG), jet aircraft fuel, [[naphtha]], [[kerosene]], heating [[fuel oil]]s, [[lubricating oil]]s, [[asphalt]] and [[petroleum coke]]. | ||
* [[Natural gas processing]] plant: Purifies and converts raw [[natural gas]] into residential, commercial and industrial fuel gas, and also recovers byproduct [[ | * [[Natural gas processing]] plant: Purifies and converts raw [[natural gas]] into residential, commercial and industrial fuel gas, and also recovers byproduct [[sulphur]] and [[natural gas liquids]] (NGL) such as [[ethane]], [[propane]], [[butane]]s and [[pentane]]s. | ||
* [[Sugarcane|Sugar refinery]]: Converts [[sugar cane]] and [[sugar beets]] into crystallized [[sugar]] and sugar syrups. | * [[Sugarcane|Sugar refinery]]: Converts [[sugar cane]] and [[sugar beets]] into crystallized [[sugar]] and sugar syrups. | ||
* [[Salt refinery]]: Converts salt ([[Sodium chloride|NaCl]]), produced by underground mining, the [[solar evaporation]] of sea water (or other water ponds) or by [[solution mining ]] and [[Vacuum evaporator|vacuum evaporation]], into crystallized salt used for cooking and the flavoring of food, as well as for various industrial uses (notably for the production of [[chlorine]]). | * [[Salt refinery]]: Converts salt ([[Sodium chloride|NaCl]]), produced by underground mining, the [[solar evaporation]] of sea water (or other water ponds) or by [[solution mining ]] and [[Vacuum evaporator|vacuum evaporation]], into crystallized salt used for cooking and the flavoring of food, as well as for various industrial uses (notably for the production of [[chlorine]]). | ||
| Line 31: | Line 31: | ||
There are a great many ways in which to configure the various unit processes used in the processing of raw natural gas. The image below is a generalized, schematic [[Process flow diagram|block flow diagram]] of a typical natural gas processing plant configuration. It shows the various unit processes used to convert raw natural gas into sales gas pipelined to the end user markets. | There are a great many ways in which to configure the various unit processes used in the processing of raw natural gas. The image below is a generalized, schematic [[Process flow diagram|block flow diagram]] of a typical natural gas processing plant configuration. It shows the various unit processes used to convert raw natural gas into sales gas pipelined to the end user markets. | ||
The block flow diagram also depicts how processing of the raw natural gas yields byproduct sulfur, byproduct ethane, and natural gas liquids (NGL) such as propane, butanes and natural gasoline (denoted as pentanes +).<ref>[http://www.eia.doe.gov/pub/oil_gas/natural_gas/feature_articles/2006/ngprocess/ngprocess.pdf Natural Gas Processing: The Crucial Link Between Natural Gas Production and Its Transportation to Market]</ref><ref>[http://www.uop.com/gasprocessing/6070.html Example Gas Plant]</ref><ref>[http://www.axens.net/upload/presentations/fichier/axens_gpagcc_2004v2.pdf From Purification to Liquefaction Gas Processing]</ref><ref>[http://www.spe.org/specma/binary/files/5804785Syn10682.pdf Feed-Gas Treatment Design for the Pearl GTL Project]</ref><ref>[http://lnglicensing.conocophillips.com/ | The block flow diagram also depicts how processing of the raw natural gas yields byproduct sulfur, byproduct ethane, and natural gas liquids (NGL) such as propane, butanes and natural gasoline (denoted as pentanes +).<ref>[http://www.eia.doe.gov/pub/oil_gas/natural_gas/feature_articles/2006/ngprocess/ngprocess.pdf Natural Gas Processing: The Crucial Link Between Natural Gas Production and Its Transportation to Market]</ref><ref>[http://www.uop.com/gasprocessing/6070.html Example Gas Plant]</ref><ref>[http://www.axens.net/upload/presentations/fichier/axens_gpagcc_2004v2.pdf From Purification to Liquefaction Gas Processing]</ref><ref>[http://www.spe.org/specma/binary/files/5804785Syn10682.pdf Feed-Gas Treatment Design for the Pearl GTL Project]</ref><ref>[http://lnglicensing.conocophillips.com/EN/publications/documents/AICHELNGNGLIntegrationPaper.pdf Benefits of integrating NGL extraction and LNG liquefaction]</ref> | ||
[[Image:NatGasProcessing.png|frame|center|584px|Schematic flow diagram of a typical natural gas processing plant]] | [[Image:NatGasProcessing.png|frame|center|584px|Schematic flow diagram of a typical natural gas processing plant]] | ||
| Line 51: | Line 51: | ||
As shown in the above schematic flow diagram,<ref>[http://www.trivenigroup.com/sugar/plant-process.html Process flow diagram for sugar refining]</ref> the juice (containing 10 – 15 percent [[sucrose]]) is heated to about 65 – 70 °C and mixed with [[lime (mineral)|lime]] and with gaseous [[sulfur dioxide]] (SO<sub>2</sub>). The lime serves to adjust the [[pH]] of the juice to about 7.0 – 7.1 which arrests sucrose's decay into [[glucose]] and [[fructose]], and precipitates out some impurities. The sulfur dioxide serves to decolorize the juice. The juice is then further heated to about 100 – 105 °C and sent through a classifier where the precipitated impurities and other solids are settled out and removed. | As shown in the above schematic flow diagram,<ref>[http://www.trivenigroup.com/sugar/plant-process.html Process flow diagram for sugar refining]</ref> the juice (containing 10 – 15 percent [[sucrose]]) is heated to about 65 – 70 °C and mixed with [[lime (mineral)|lime]] and with gaseous [[sulfur dioxide]] (SO<sub>2</sub>). The lime serves to adjust the [[pH]] of the juice to about 7.0 – 7.1 which arrests sucrose's decay into [[glucose]] and [[fructose]], and precipitates out some impurities. The sulfur dioxide serves to decolorize the juice. The juice is then further heated to about 100 – 105 °C and sent through a classifier where the precipitated impurities and other solids are settled out and removed. | ||
The clarified juice is next concentrated in a [[multiple-effect evaporator]] to make a syrup with about 60 – 65 weight percent sucrose. The syrup is again treated sulfur dioxide for further decolorization and then is further concentrated under [[vacuum]] until it becomes [[supersaturated]] with sugar. Upon cooling, sugar crystallizes out of the syrup. | The clarified juice is next concentrated in a [[multiple-effect evaporator]] to make a syrup with about 60 – 65 weight percent sucrose. The syrup is again treated sulfur dioxide for further decolorization and then is further concentrated under [[Vacuum (science)|vacuum]] until it becomes [[supersaturated]] with sugar. Upon cooling, sugar crystallizes out of the syrup. | ||
The crystallized sugar is separated from the residual liquid syrup (''[[molasses]]'') by [[Centrifuge|centrifuging]]. The end product is a white, crystalline sugar referred to as ''mill white'', ''plantation white'' or ''crystal sugar''. To produce ''[[granulated sugar]]'', in which the individual sugar grains do not clump together, sugar must be dried. Drying is accomplished by first drying the sugar in a hot [[rotary dryer]], and then by blowing cool air through it for several days. | The crystallized sugar is separated from the residual liquid syrup (''[[molasses]]'') by [[Centrifuge|centrifuging]]. The end product is a white, crystalline sugar referred to as ''mill white'', ''plantation white'' or ''crystal sugar''. To produce ''[[granulated sugar]]'', in which the individual sugar grains do not clump together, sugar must be dried. Drying is accomplished by first drying the sugar in a hot [[rotary dryer]], and then by blowing cool air through it for several days. | ||
| Line 70: | Line 70: | ||
{|border="0" align="center" | {|border="0" align="center" | ||
|- | |- | ||
|valign="top" |{{Image|SF Bay Salt Ponds.jpg|right|240px|Salt ponds in the [[San Francisco Bay]], [[California]].}} | |valign="top" |{{Image|SF Bay Salt Ponds.jpg|right|240px|Salt ponds in the [[San Francisco Bay]], [[California (U.S. state)|California]].}} | ||
|valign="top" |{{Image|Inca salt ponds.jpg|right|240px|[[Inca]] salt ponds in [[Peru]].}} | |valign="top" |{{Image|Inca salt ponds.jpg|right|240px|[[Inca]] salt ponds in [[Peru]].}} | ||
|valign="top" |{{Image|Dead Sea salt.jpg|right|215px|Salt in the [[Dead Sea]], [[Ein Gadi]], [[Israel]].}} | |valign="top" |{{Image|Dead Sea salt.jpg|right|215px|Salt in the [[Dead Sea]], [[Ein Gadi]], [[Israel]].}} | ||
| Line 142: | Line 142: | ||
==References== | ==References== | ||
<references/> | <references/>[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 16:00, 10 October 2024
Refineries are industrial manufacturing facilities composed of a group of chemical engineering unit processes and unit operations[1][2] used for the conversion certain raw materials such as petroleum crude oil, mined ores, sugar or salt into finished products of value or for the refining and purification of partially converted raw materials into finished products.
Types of refineries
The various types of refineries include:
- Petroleum refinery: Converts petroleum crude oil into high-octane motor fuel (gasoline/petrol), diesel oil, liquefied petroleum gases (LPG), jet aircraft fuel, naphtha, kerosene, heating fuel oils, lubricating oils, asphalt and petroleum coke.
- Natural gas processing plant: Purifies and converts raw natural gas into residential, commercial and industrial fuel gas, and also recovers byproduct sulphur and natural gas liquids (NGL) such as ethane, propane, butanes and pentanes.
- Sugar refinery: Converts sugar cane and sugar beets into crystallized sugar and sugar syrups.
- Salt refinery: Converts salt (NaCl), produced by underground mining, the solar evaporation of sea water (or other water ponds) or by solution mining and vacuum evaporation, into crystallized salt used for cooking and the flavoring of food, as well as for various industrial uses (notably for the production of chlorine).
- Various metal refineries converting metallic ores into end product metals such as alumina, copper, gold, lead, nickel, silver, uranium, and zinc.
A typical petroleum refinery
Petroleum refineries are very large industrial complexes that involve a great many different processing units and auxiliary facilities such as utility units and storage tanks. Each refinery has its own unique arrangement and combination of refining processes largely determined by the refinery location, desired products and economic considerations. There are most probably no two refineries that are identical in every respect.
The image below is a schematic flow diagram of a typical oil refinery that depicts the various unit processes and the flow of intermediate product streams that occurs between the inlet crude oil feedstock and the final end products. The diagram depicts only one of the literally hundreds of different oil refinery configurations. It does not include any of the usual refinery facilities providing utilities such as steam, cooling water, and electric power as well as storage tanks for crude oil feedstock and for intermediate products and end products.[3][4][5][6]
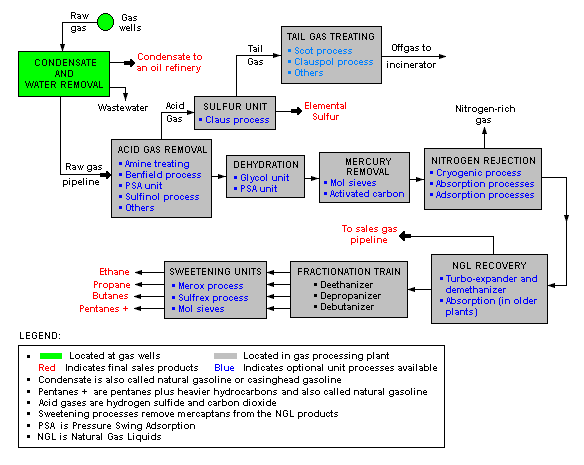
A typical natural gas processing plant
There are a great many ways in which to configure the various unit processes used in the processing of raw natural gas. The image below is a generalized, schematic block flow diagram of a typical natural gas processing plant configuration. It shows the various unit processes used to convert raw natural gas into sales gas pipelined to the end user markets.
The block flow diagram also depicts how processing of the raw natural gas yields byproduct sulfur, byproduct ethane, and natural gas liquids (NGL) such as propane, butanes and natural gasoline (denoted as pentanes +).[7][8][9][10][11]
Typical refining of sugar
Most of the sugar produced worldwide is derived either from sugar cane or sugar beets. However, the sugar produced from sugar cane is at least twice the amount produced by sugar beets. For that reason, this section deals with sugar produced from sugar cane.
The refining of sugar cane into sugar is usually done in two stages. The first stage is the preparation and milling of freshly harvested sugar cane. In some cases, the preparation and milling may be done locally where the sugar cane is grown and harvested. In the milling stage, the sugar cane is first washed, chopped, and shredded by revolving knives. Then the shredded cane is mixed with water and crushed to produce a sugar juice.
As shown in the above schematic flow diagram,[12] the juice (containing 10 – 15 percent sucrose) is heated to about 65 – 70 °C and mixed with lime and with gaseous sulfur dioxide (SO2). The lime serves to adjust the pH of the juice to about 7.0 – 7.1 which arrests sucrose's decay into glucose and fructose, and precipitates out some impurities. The sulfur dioxide serves to decolorize the juice. The juice is then further heated to about 100 – 105 °C and sent through a classifier where the precipitated impurities and other solids are settled out and removed.
The clarified juice is next concentrated in a multiple-effect evaporator to make a syrup with about 60 – 65 weight percent sucrose. The syrup is again treated sulfur dioxide for further decolorization and then is further concentrated under vacuum until it becomes supersaturated with sugar. Upon cooling, sugar crystallizes out of the syrup.
The crystallized sugar is separated from the residual liquid syrup (molasses) by centrifuging. The end product is a white, crystalline sugar referred to as mill white, plantation white or crystal sugar. To produce granulated sugar, in which the individual sugar grains do not clump together, sugar must be dried. Drying is accomplished by first drying the sugar in a hot rotary dryer, and then by blowing cool air through it for several days.
The refining process described in this section is sometimes referred to as the Double Sulfation (DS) process. There are other refining processes that use calcium phosphate (instead of lime) to remove impurities from the sugar juice (and/or the sugar syrup) and treatment with activated carbon (rather than gaseous sulfur dioxide) for decolorization.
The fibrous solids, called bagasse, remaining after the crushing of the shredded sugar cane, are burned for fuel within the sugar refinery. Any surplus bagasse can be used for animal feed, in paper manufacture, or burned to generate electricity for the local power grid.
Salt production and refining
There are three different methods used for the production of salt: solar evaporation of saline water, underground salt mining and mechanical evaporation of solution mined salt.[13][14][15]
Solar evaporation and refining
Solar salt (sodium chloride or NaCl) is produced by using sunlight and wind to evaporate saline ocean water, open water ponds or saline lakes. This is the oldest method of producing salt and has been used since salt crystals were first observed in trapped ponds of seawater. It requires a climate where there are steady prevailing winds and the evaporation rate of open water exceeds the rate of rainfall for long time periods. Some examples of solar salt production are:
The water in the San Francisco Bay salt ponds is not as saline (i.e., salty) as the Pacific Ocean seawater because it is diluted by fresh water from local rivers, creeks and other sources. The bay water first flows into the intake set of ponds and is subsequently moved progressively through other ponds. In the first stage of ponds, evaporation over time reduces the water volume by about 70 percent which increases the salinity and impurities such as calcium sulfate (CaSO4) precipitate. As the water is subsequently moved through the other pond stages, other impurities precipitate out and 95 percent of the water is eventually evaporated before the water is moved into the final ponds (referred to as the crystallizing ponds) where the sodium chloride precipitates out at about 40 tons per acre (90 tonnes per hectare) and is then mechanically harvested.[16][17][18] It takes up to 5 years for the sun and wind to evaporate the bay water and to harvest the salt.
The colors of the salt ponds reflect the interaction of plants, aquatic life and varying salinity. In the low-salinity ponds, algae create a green color. In the moderate-salinity ponds, Dunaliella algae proliferate and the color of this ponds become a lighter shade of green. In the mid-salinity ponds, tiny bay shrimp produce an orange color. In the high-salinity ponds, the Dunaliella algae produce a red color and halophilic bacteria (i.e., organisms requiring a salty environment) contribute a purplish-red color.[16]
To produce high-purity salt (99.9 percent pure) for use in cooking and flavoring of foods, the harvested solar salt must be further purified in a salt refinery. In the refinery, the solar salt is first washed, which removes dust or other debris and some trace minerals that may have clung to the salt crystals during the harvesting of the solar salt. The cleansed solar salt is then dissolved in tanks of pure drinking water.[18]
The salt solution (i.e., brine) from the tanks is then passed through vacuum evaporators, which remove the water and crystallize the salt. After the salt re-crystallizes, it is dried, filtered, and air-cooled. A series of vibrating screens is used to segregate the dried crystals into various sizes for packaging and shipment to end-users.[18]
Underground mining of rock salt
Rock salt (referred to as halite) is mined from underground deposits reached through a circular shaft, usually about 20 feet (6 metre) in diameter and as deep as 2,000 feet (610 metre), depending on the depth and location of the salt deposit.[19]
Mining methods depend on whether the salt deposit is layered horizontally or is a vertical salt dome. Layered deposits are mined using the room and pillar method in which drilling and blasting creates horizontal rooms of 10 – 25 feet (3 – 8 metre) high and about 50 feet (15 metre) wide. Openings or crosscuts are created perpendicular to the length of the rooms to connect the rooms at planned intervals. Salt pillars are left in place to provide structural support for the overlying roof and other layers. Most room-and-pillar mines recover about 45 – 65% of the salt available, with the remainder left behind as pillar supports with margins both above and below the mined area. Several rooms may be blasted each day with each blast bringing down 350 – 900 tons (317 – 816 tonnes).[19]
For salt dome deposits, a level of room-and-pillar extraction is first completed and the usual practice is to then bench the mine by drilling and blasting the floor extending the excavation downward and removing vast quantities with each blast.[19]
Typically, salt mining uses large, diesel-powered equipment for cutting, drilling, blasting and loading the blasted salt onto diesel-powered trucks which transport the salt to crushers. Conveyor belts then carry the crushed salt to the a hoist. Each hoist can lift 18 – 20 tons (16 – 18 tonnes). Large salt mines may hoist up to 900 tons (816 tonnes) an hour.[19]
Once the salt has been hoisted to above ground, vibrating screens are used to separate the mined salt into various sizes and each size is conveyed to its designated storage bins. From the storage bins, the salt is subsequently shipped either to various end-user buyers of mined rock salt or to salt refineries for final purification (as described above for solar salt).
Solution mining and evaporation
In solution mining, vertical wells are drilled into underground layered salt deposits or into vertical salt dome deposits. The wells are used to inject fresh or recycled water into the salt deposits to dissolve the salt, with a residence time long enough for the resulting solution (brine) to become essentially saturated with salt.[14][20]
Some solution mines consist of a single well using one tube within a large tube. Water is injected through the inner tubing and the salt brine is withdrawn through the annular space between the inner tube and the outer tube. Other solution mines have multiple wells, some for water injection and some for brine withdrawal.[14][20]
Once the brine is withdrawn aboveground, it is processed in salt refinery using vacuum evaporation to produce crystallized salt (as described above for solar salt).
The size and shape of solution-mined caverns can be measured and controlled with well logging devices and well operating techniques that minimize the potential for surface subsidence. After the end of their use for producing salt, many solution-mined caverns have been used to store natural gas and other materials.
Many chlor-alkali process plants produce chlorine (Cl2), hydrogen (H2) and sodium hydroxide (NaOH) by the electrolysis of salt brine have their own captive solution mines to produce the brine.
The equipment used in refineries
Refineries utilize a great many different types of physical equipment such as:
References
- ↑ McCabe, W., Smith, J. and Harriott, P. (2004). Unit Operations of Chemical Engineering, 7th Edition. McGraw-Hill. ISBN 0-07-284823-5.
- ↑ Perry, R.H. and Green, D.W. (Editors) (2007). Perry's Chemical Engineers' Handbook, 8th Edition. McGraw-Hill. ISBN 0-07-142294-3.
- ↑ Gary, J.H. and Handwerk, G.E. (1984). Petroleum Refining Technology and Economics, 2nd Edition. Marcel Dekker, Inc. ISBN 0-8247-7150-8.
- ↑ Guide to Refining from Chevron Oil's website
- ↑ Refinery flowchart from Universal Oil Products' website
- ↑ An example flowchart of fractions from crude oil at a refinery
- ↑ Natural Gas Processing: The Crucial Link Between Natural Gas Production and Its Transportation to Market
- ↑ Example Gas Plant
- ↑ From Purification to Liquefaction Gas Processing
- ↑ Feed-Gas Treatment Design for the Pearl GTL Project
- ↑ Benefits of integrating NGL extraction and LNG liquefaction
- ↑ Process flow diagram for sugar refining
- ↑ Salt production technologies
- ↑ 14.0 14.1 14.2 Salt Production and Processing
- ↑ How Salt Is Made
- ↑ 16.0 16.1 Turning Salt Into Environmental Gold
- ↑ Solar Salt
- ↑ 18.0 18.1 18.2 Virtual tour of Cargill's San Francisco Bay salt works
- ↑ 19.0 19.1 19.2 19.3 Rock salt (halite)
- ↑ 20.0 20.1 Solution mining