Glutamic acid: Difference between revisions
imported>David E. Volk mNo edit summary |
mNo edit summary |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
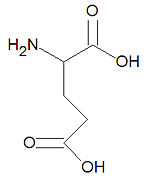
{{Image|Glutamic acid stick figure.jpg|right|150px|'''Glutamic acid''', one of the common alpha-amino acids.}} | |||
'''Glutamic acid''', also called '''glutamate''' and abbreviated as '''Glu''' or '''E''', is one of the twenty common [[amino acid]]s used by living organisms to build [[protein]]s. It is one of only two acidic amino acids, the other being [[aspartic acid]], and it is similar to [[glutamine]] which has an amide function in place of the acid. Being [[hydrophilic]], glutamate is often found on the surfaces of proteins. Glutamate also serves as an energy source in the brain, is the chemical precursor for the amino acids glutamine, [[proline]] and [[arginine]], and most of the amino acids derive their <math>\alpha</math>-amino nitrogen atom from the glutamate <math>\alpha</math>-amino nitrogen by transamination. Glutamate is a major [[neurotransmitter]] in the brain. | '''Glutamic acid''', also called '''glutamate''' and abbreviated as '''Glu''' or '''E''', is one of the twenty common [[amino acid]]s used by living organisms to build [[protein]]s. It is one of only two acidic amino acids, the other being [[aspartic acid]], and it is similar to [[glutamine]] which has an amide function in place of the acid. Being [[hydrophilic]], glutamate is often found on the surfaces of proteins. Glutamate also serves as an energy source in the brain, is the chemical precursor for the amino acids glutamine, [[proline]] and [[arginine]], and most of the amino acids derive their <math>\alpha</math>-amino nitrogen atom from the glutamate <math>\alpha</math>-amino nitrogen by transamination. Glutamate is a major [[neurotransmitter]] in the brain. | ||
| Line 9: | Line 9: | ||
::'''NH<sub>4</sub><sup>+</sup> + <math>\alpha</math>-ketoglutarate + [[NADPH]] + H<sup>+</sup> → L-glutamate + NADP<sup>+</sup> + H<sub>2</sub>0''' | ::'''NH<sub>4</sub><sup>+</sup> + <math>\alpha</math>-ketoglutarate + [[NADPH]] + H<sup>+</sup> → L-glutamate + NADP<sup>+</sup> + H<sub>2</sub>0''' | ||
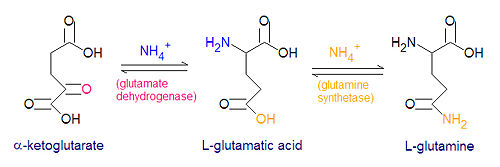
{{Image|Glutamate glutamine synth1.jpg|center|500px|Synthesis of glutamic acid and glutamine. Note that the neutral forms of the chemicals are shown here rather that the deprotonated acid functions and protonated amine groups. }} | |||
Three amino acids are derived from glutamate, namely glutamine, proline and arginine. The enzyme [[glutamine synthetase]] catalyzes the reaction between glutamate and ammonium ion, with energy derived from the hydrolysis of ATP. Nitrogen metabolism is largely controlled by glutamine synthetase and [[glutamate dehydrogenase]] which are present in all living organisms, and most procaryotes also have the enzyme [[glutamate synthase]] to catalyze the reductive amination of <math>\alpha</math>-ketoglutarate. | Three amino acids are derived from glutamate, namely glutamine, proline and arginine. The enzyme [[glutamine synthetase]] catalyzes the reaction between glutamate and ammonium ion, with energy derived from the hydrolysis of ATP. Nitrogen metabolism is largely controlled by glutamine synthetase and [[glutamate dehydrogenase]] which are present in all living organisms, and most procaryotes also have the enzyme [[glutamate synthase]] to catalyze the reductive amination of <math>\alpha</math>-ketoglutarate. | ||
| Line 19: | Line 19: | ||
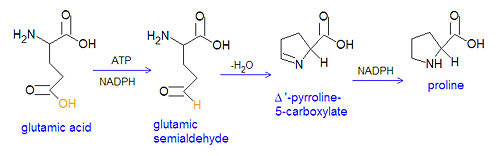
The non-essential amino acids proline and arginine are derived from glutamate through several steps. First, glutamate is converted to an [[acyl phosphate]] by reacting with ATP, and the acyl phosphate is reduced to [[glutamatic-γ-semialdehyde]] by NADPH. The semialdehyde can be converted to ornithine, which is a precursor of arginine, or it can cyclize to from Δ'-[[Pyrroline-5-carboxylate]], which can be reduced by NADPH to from proline. | The non-essential amino acids proline and arginine are derived from glutamate through several steps. First, glutamate is converted to an [[acyl phosphate]] by reacting with ATP, and the acyl phosphate is reduced to [[glutamatic-γ-semialdehyde]] by NADPH. The semialdehyde can be converted to ornithine, which is a precursor of arginine, or it can cyclize to from Δ'-[[Pyrroline-5-carboxylate]], which can be reduced by NADPH to from proline. | ||
{{Image|Proline synthesis.jpg|center|500px|Biosynthesis of proline via glutamate semialdehyde and pyrroline. Note that the neutral forms of the chemicals are shown, rather than deprotonated acid groups and protonated amino groups.}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 12:01, 22 August 2024
Glutamic acid, also called glutamate and abbreviated as Glu or E, is one of the twenty common amino acids used by living organisms to build proteins. It is one of only two acidic amino acids, the other being aspartic acid, and it is similar to glutamine which has an amide function in place of the acid. Being hydrophilic, glutamate is often found on the surfaces of proteins. Glutamate also serves as an energy source in the brain, is the chemical precursor for the amino acids glutamine, proline and arginine, and most of the amino acids derive their -amino nitrogen atom from the glutamate -amino nitrogen by transamination. Glutamate is a major neurotransmitter in the brain.
Synthesis and conversion of glutamic acid
The enzyme glutamate dehydrogenase catalyzes the reaction between an ammonium ion and -ketoglutarate to form L-glutamic acid. This reaction is reversible.
- NH4+ + -ketoglutarate + NADPH + H+ → L-glutamate + NADP+ + H20
Three amino acids are derived from glutamate, namely glutamine, proline and arginine. The enzyme glutamine synthetase catalyzes the reaction between glutamate and ammonium ion, with energy derived from the hydrolysis of ATP. Nitrogen metabolism is largely controlled by glutamine synthetase and glutamate dehydrogenase which are present in all living organisms, and most procaryotes also have the enzyme glutamate synthase to catalyze the reductive amination of -ketoglutarate.
- glutamate + NH4+ + ATP → glutamine + ADP + Pi + H+
- -ketoglutarate + glutamine + NADPH + H+ → 2 glutamate + NADP+
The non-essential amino acids proline and arginine are derived from glutamate through several steps. First, glutamate is converted to an acyl phosphate by reacting with ATP, and the acyl phosphate is reduced to glutamatic-γ-semialdehyde by NADPH. The semialdehyde can be converted to ornithine, which is a precursor of arginine, or it can cyclize to from Δ'-Pyrroline-5-carboxylate, which can be reduced by NADPH to from proline.