Azole: Difference between revisions
imported>Caesar Schinas m (Bot: Update image code) |
mNo edit summary |
||
| (One intermediate revision by one other user not shown) | |||
| Line 8: | Line 8: | ||
[[Image:Lanosterol ergosterol.jpg|right|thumb|250px]] | [[Image:Lanosterol ergosterol.jpg|right|thumb|250px]] | ||
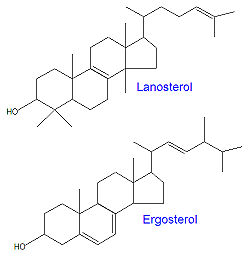
A number of drugs that are used to treat fungal infections are active due to the presence of one or more azole (or triazole) rings, often imidazole in particular. Examples of such drugs include [[econazole]], [[fluconazole]], [[itraconazole]], [[clotrimazole]], [[ketoconazole]] and [[miconazole]]. The triazole drugs fluconazole and itraconazole have greater affinity for fungi and therefore lead to fewer side effects compared to the drugs based on only one azole ring system. The azole-based antifungal drugs work by inhibiting [[14-alpha-demethylase]], a [[P450]] enzyme required in the conversion of [[lanosterol]] to [[ergosterol]]. | A number of drugs that are used to treat fungal infections are active due to the presence of one or more azole (or triazole) rings, often imidazole in particular. Examples of such drugs include [[econazole]], [[fluconazole]], [[itraconazole]], [[clotrimazole]], [[ketoconazole]] and [[miconazole]]. The triazole drugs fluconazole and itraconazole have greater affinity for fungi and therefore lead to fewer side effects compared to the drugs based on only one azole ring system. The azole-based antifungal drugs work by inhibiting [[14-alpha-demethylase]], a [[P450]] enzyme required in the conversion of [[lanosterol]] to [[ergosterol]].[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:00, 15 July 2024
An azole is a chemical compound containing a five-membered aromatic ring structure with two heteroatoms, at least one of which must be a nitrogen atom. One, and only one, lone pair of electrons from each heteroatom is part of the aromatic bonding in an azole. Examples of common azole compounds include pyrazole, imidazole, thiazole, oxazole and isoxazole, shown in the illustration. Some antifungal drugs are based on the azole structure (see Catalog of azole-based antifungal drugs). The name or suffix azole changes upon saturation of the double bonds. When one of the double bonds becomes saturated, the resulting compound name changes its ending from azole to azoline, and further reduction of the second double bond produces an azolidine. Thus, the saturation of one double bond of oxazole yields 2-oxazoline and a further reduction yields oxazolidine. Six-membered aromatic heterocyclic systems with two nitrogens exist, and two of them, pyrimidine and purine, are particularly important biochemicals. The numbering of ring atoms in azoles starts with the heteroatom that is not part of a double bond, and then proceeds towards the other heteroatom.
The triazoles are two isomers, namely 1,2,3-triazole or 1,2,4-triazole, with the formula C2H3N3. That is, they are similar to pyrazole and imadazole shown above, but they would have an additional nitrogen atom in the ring structure.
A number of drugs that are used to treat fungal infections are active due to the presence of one or more azole (or triazole) rings, often imidazole in particular. Examples of such drugs include econazole, fluconazole, itraconazole, clotrimazole, ketoconazole and miconazole. The triazole drugs fluconazole and itraconazole have greater affinity for fungi and therefore lead to fewer side effects compared to the drugs based on only one azole ring system. The azole-based antifungal drugs work by inhibiting 14-alpha-demethylase, a P450 enzyme required in the conversion of lanosterol to ergosterol.