Hetacillin: Difference between revisions
imported>David E. Volk (chem infobox etc) |
mNo edit summary |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{Chem infobox | {{Chem infobox | ||
|align=right | |align=right | ||
| Line 27: | Line 26: | ||
== External links == | == External links == | ||
{{CZMed}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 16:00, 27 August 2024
|
| |||||||
| hetacillin | |||||||
| |||||||
| Uses: | antibiotic drug | ||||||
| Properties: | beta-lactam | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
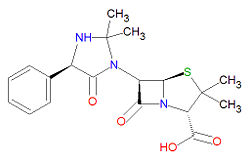
Hetacillin is a penicillin-like, beta-lactam-based antibiotic prodrug used to treat infections, usually from Gram-positive bacteria. It is sold under the brand names Hetacillin potassium®, Versapen® and Versapen-k®.
Mechanism of action
Hetacillin is a prodrug with no antibacterial activity, but it is metabolized into the antibiotic ampicillin. Hetacillin is prepared by reacting ampicillin with acetone because ampicillin is much less stable towards ring-opening reactions. Once converted to ampicillin, the ampicillin interferes with the final stage of cell wall synthesis by binding to penicillin-binding proteins, leading to autolysis of the bacteria by autolysin enzymes.
Chemistry
It is a beta-lactam structure, and its chemical name is (2S,5R,6R)-6-[(4R)-2,2-dimethyl-5-oxo-4-phenylimidazolidin-1-yl]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid. Its chemical formula is C19H23N3O4S (MW = 389.4686 g/mol). Hetacillin is susceptible to degradation in bacteria that produce beta-lactamase.
External links
The most up-to-date information about Hetacillin and other drugs can be found at the following sites.
- Hetacillin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Hetacillin - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Hetacillin - Detailed information from DrugBank.