Triazole: Difference between revisions
imported>David E. Volk No edit summary |
mNo edit summary |
||
| (13 intermediate revisions by 4 users not shown) | |||
| Line 13: | Line 13: | ||
|hazards= | |hazards= | ||
|iupac= see below | |iupac= see below | ||
|casnumber= 288-88-0; | |casnumber= 288-88-0;288-36-8 | ||
}} | }} | ||
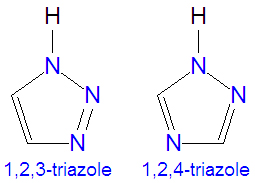
The [[triazole]]s are two isomers, namely 1,2,3-triazole or 1,2,4-triazole, with the formula C<sub>2</sub>H<sub>3</sub>N<sub>3</sub>. They are [[aromatic]] ring compounds | The [[triazole]]s are two isomers, namely 1,2,3-triazole or 1,2,4-triazole, with the formula C<sub>2</sub>H<sub>3</sub>N<sub>3</sub>. They are [[aromatic]] ring compounds similar to the [[azole]]s [[pyrazole]] and [[imidazole]], but with an additional nitrogen atom in the ring structure. Like the azoles, triazoles are used in many antifungal drugs and fungicides, and the triazole-based drugs are more selective for fungi than mammalian cells than the azole-based antifungal compounds. Today, most substituted triazoles are produced by so-called [[click chemistry]] pioneered by [[K. Barry Sharpless]] and others (see below). Synonyms for both of these triazoles sometimes denote that a proton is attached in the 1-position, as for example, the naming 1H-1,2,3-triazole or 1,2,3-1H-triazole. | ||
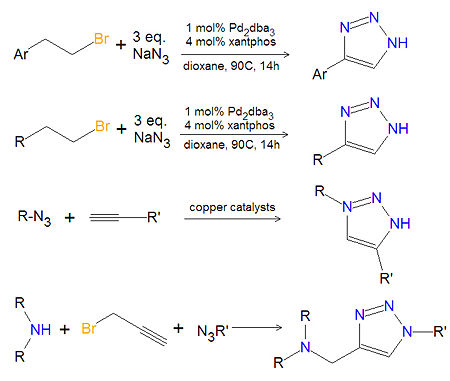
== | == Synthesis of substituted 1,2,3-triazoles == | ||
Most substituted triazoles are synthesized by | Most substituted triazoles are synthesized by one of several click chemistry methods. One method uses [[palladium]] (Pd) catalysts to couple [[alkyne|alkenyl halides]] and [[azide|sodium azide]] to product 4-substituted-1,2,3-triazoles<ref>{{cite journal|title=Developments in Pd catalysis: synthesis of 1H-1,2,3-triazoles from sodium azide and alkenyl bromides| | ||
author=Barluenga J ''et al.''|journal=Angew Chem Int Ed|year=2006|volume=45|pages=6893-6|doi=10.1002/anie.200601045}}</ref>. This reaction also works with aromatic alkenyl halides. Click chemistry between azides and terminal aklynes, catalyzed by various [[copper]] reagents, can be used to synthesize 3,5-disubstituted-1,2,3-triazoles<ref>{{cite journal|title=Huisgen 1,3-Dipolar Cycloaddition |author=Rostovtsev VV ''et al.''|journal=Angew Chem|volume=114|year=2002|pages=2708-11|doi=10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0}}</ref>,<ref>{{cite journal|title=Heterogeneous copper-in-charcoal-catalyzed click chemistry|author=Lipshutz BH, Taft BR|journal=Angew Chem Int Ed|year=2006|volume=45|pages=8235-8|doi=10.1002/anie.200603726}}</ref>. | |||
{{Image|1,2,3-triazole synthesis substituted.jpg|right|450px|Triazole synthesis.}} | |||
A variety of 1,4-disubstituted-1,2,3-triazoles can be made economically and in an environmentally friendly manner, by reacting a secondary amine, propargyl halides and an azide together in water, under atmospheric oxygen with a copper | A variety of 1,4-disubstituted-1,2,3-triazoles can be made economically and in an environmentally friendly manner, by reacting a secondary amine, propargyl halides and an azide together in water, under atmospheric oxygen with a copper catalyst.<ref>{{cite journal|title=Efficient synthesis of 1,4-disubstituted 1,2,3-triazoles in ionic liquid/water system|author=Yan ''et al.''|journal=Tetrahedron|year=2005|volume=61|pages=9331-7|doi=10.1016/j.tetlet.2006.01.004}}</ref>. Another method of 1,4-disubstituted-1,2,3-triazoles (not shown) uses no solvent. In this method, the cycloaddition of alkyl azides with enol ethers provides triazoles that are not amendable to synthesis using the coupling of azides with alkynes.<ref>{{cite journal|title=Synthesis of 1,2,3-triazoles by cycloadditions of azides with enol ethers|author=Rogue DR ''et al.'' |journal=Synthesis|year=2005|volume=2005|pages=2497-2502|doi=10.1055/s-2005-872116}}</ref> A variety of other synthetic methods are available<ref>{{cite web|url=http://www.organic-chemistry.org/heterocycles/1,2,3-triazoles.shtm|title=1,2,3-triazole synthesis|accessdate=2008-05-17}}</ref>. | ||
| Line 29: | Line 30: | ||
== References == | == References == | ||
<references/> | <references/> | ||
[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 16:00, 30 October 2024
|
| |||||||
| triazole | |||||||
| |||||||
| Uses: | antifungal | ||||||
| Properties: | basic | ||||||
| Hazards: | |||||||
| |||||||
The triazoles are two isomers, namely 1,2,3-triazole or 1,2,4-triazole, with the formula C2H3N3. They are aromatic ring compounds similar to the azoles pyrazole and imidazole, but with an additional nitrogen atom in the ring structure. Like the azoles, triazoles are used in many antifungal drugs and fungicides, and the triazole-based drugs are more selective for fungi than mammalian cells than the azole-based antifungal compounds. Today, most substituted triazoles are produced by so-called click chemistry pioneered by K. Barry Sharpless and others (see below). Synonyms for both of these triazoles sometimes denote that a proton is attached in the 1-position, as for example, the naming 1H-1,2,3-triazole or 1,2,3-1H-triazole.
Synthesis of substituted 1,2,3-triazoles
Most substituted triazoles are synthesized by one of several click chemistry methods. One method uses palladium (Pd) catalysts to couple alkenyl halides and sodium azide to product 4-substituted-1,2,3-triazoles[1]. This reaction also works with aromatic alkenyl halides. Click chemistry between azides and terminal aklynes, catalyzed by various copper reagents, can be used to synthesize 3,5-disubstituted-1,2,3-triazoles[2],[3].
A variety of 1,4-disubstituted-1,2,3-triazoles can be made economically and in an environmentally friendly manner, by reacting a secondary amine, propargyl halides and an azide together in water, under atmospheric oxygen with a copper catalyst.[4]. Another method of 1,4-disubstituted-1,2,3-triazoles (not shown) uses no solvent. In this method, the cycloaddition of alkyl azides with enol ethers provides triazoles that are not amendable to synthesis using the coupling of azides with alkynes.[5] A variety of other synthetic methods are available[6].
References
- ↑ Barluenga J et al. (2006). "Developments in Pd catalysis: synthesis of 1H-1,2,3-triazoles from sodium azide and alkenyl bromides". Angew Chem Int Ed 45: 6893-6. DOI:10.1002/anie.200601045. Research Blogging.
- ↑ Rostovtsev VV et al. (2002). "Huisgen 1,3-Dipolar Cycloaddition". Angew Chem 114: 2708-11. DOI:<2708::AID-ANGE2708>3.0.CO;2-0 10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0. <2708::AID-ANGE2708>3.0.CO;2-0 Research Blogging.
- ↑ Lipshutz BH, Taft BR (2006). "Heterogeneous copper-in-charcoal-catalyzed click chemistry". Angew Chem Int Ed 45: 8235-8. DOI:10.1002/anie.200603726. Research Blogging.
- ↑ Yan et al. (2005). "Efficient synthesis of 1,4-disubstituted 1,2,3-triazoles in ionic liquid/water system". Tetrahedron 61: 9331-7. DOI:10.1016/j.tetlet.2006.01.004. Research Blogging.
- ↑ Rogue DR et al. (2005). "Synthesis of 1,2,3-triazoles by cycloadditions of azides with enol ethers". Synthesis 2005: 2497-2502. DOI:10.1055/s-2005-872116. Research Blogging.
- ↑ 1,2,3-triazole synthesis. Retrieved on 2008-05-17.