Desloratadine: Difference between revisions
imported>David E. Volk No edit summary |
mNo edit summary |
||
| (One intermediate revision by one other user not shown) | |||
| Line 8: | Line 8: | ||
|molformula= C<sub>19</sub>H<sub>19</sub>Cl<sub>4</sub>N<sub>2</sub> | |molformula= C<sub>19</sub>H<sub>19</sub>Cl<sub>4</sub>N<sub>2</sub> | ||
|molmass= 310.8 | |molmass= 310.8 | ||
|uses= | |uses=rhinitis, urticaria, puritis, hives | ||
|properties= | |properties=tricyclic histamine antagonist | ||
|hazards= | |hazards= | ||
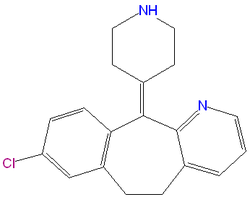
|iupac= 8-chloro-6,11-dihydro-11-(4-piperdinylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine | |iupac= 8-chloro-6,11-dihydro-11-(4-piperdinylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine | ||
|casnumber= | |casnumber= | ||
| Line 34: | Line 34: | ||
<references/> | <references/> | ||
{{CZMed}} | {{CZMed}} | ||
</div> | </div>[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:01, 6 August 2024
|
| |||||||
| Desloratadine | |||||||
| |||||||
| Uses: | rhinitis, urticaria, puritis, hives | ||||||
| Properties: | tricyclic histamine antagonist | ||||||
| Hazards: | |||||||
| |||||||
Desloratadine (Clarinex) is a light blue tablet used as an anahistamine and are available by prescription only in the United States. Each tablet contains 5 milligrams desloratadine which are taken orally (by mouth). The powder is white to off-white and is slightly soluble in water. It is very soluble in ethanol andpropylene glycol. The molecular weight for Clarinex (Desloratadine) is 310.8. It is a long acting tricyclic histamine antagonist with selective H1-receptor histamine antagonist activity.
Usage
Patients 12 and older can take 5 milligrams of Clarinex once daily to help alleviate allergic rhinitis symptoms including both nasal and non-nasal symptoms. Clarinex can also be used to treat hives (uticaria).
Pregnancy and lactation
Clarinex is classified as a class C drug by the Federal Drug Administration. The drug does pass through breast milk.
Reported adverse affects
Clarinex had a 2 percent or greater incidence of the following adverse side affects which include: pharyngitis, dry mouth, myalgia, fatigue, somnolence, dysmenorrhea in that order.
Overdose
Follow standard treatments to remove the unabsorbed drug and provide supportive care. Clarinex is not removed through hemodyalisis. [1]
References
- ↑ Clarinex (Desloratadine) (PDF). Retrieved on 2011-05-22.
The most up-to-date information about Desloratadine and other drugs can be found at the following sites.
- Desloratadine - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Desloratadine - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Desloratadine - Detailed information from DrugBank.