Crystal: Difference between revisions
imported>Caesar Schinas m (replace otheruses by dambigbox) |
imported>Meg Taylor m (spelling: occuring -> occurring) |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{dambigbox}} | {{dambigbox}} | ||
A '''crystal''' is a [[solid]] in which the constituent atoms are arranged in an orderly, repeating pattern, as distinct from the arrangement of atoms in an [[amorphous solid]]. The repeating polyhedral pattern of atoms creates preferred [[cleavage]] planes, and affects the mechanical and optical properties of the material. Although naturally | A '''crystal''' is a [[solid]] in which the constituent atoms are arranged in an orderly, repeating pattern, as distinct from the arrangement of atoms in an [[amorphous solid]]. The repeating polyhedral pattern of atoms creates preferred [[cleavage]] planes, and affects the mechanical and optical properties of the material. Although naturally occurring crystals tend to be made up of only a few kinds of atoms, very large biomolecules, like [[protein]]s and [[DNA]] can be made to form cystals under the right conditions. | ||
The structure of a crystal depends on the size and chemical properties of the constituent atoms, and can take many different forms. These forms are categorized in several ways, depending on the geometry or the chemistry of the structures. The most ordered forms, i.e., the forms with highest [[symmetry]], are cubic and hexagonal, but many variations of these structures and many other structures also exist. | The structure of a crystal depends on the size and chemical properties of the constituent atoms, and can take many different forms. These forms are categorized in several ways, depending on the geometry or the chemistry of the structures. The most ordered forms, i.e., the forms with highest [[symmetry]], are cubic and hexagonal, but many variations of these structures and many other structures also exist. | ||
Revision as of 02:42, 10 February 2010
A crystal is a solid in which the constituent atoms are arranged in an orderly, repeating pattern, as distinct from the arrangement of atoms in an amorphous solid. The repeating polyhedral pattern of atoms creates preferred cleavage planes, and affects the mechanical and optical properties of the material. Although naturally occurring crystals tend to be made up of only a few kinds of atoms, very large biomolecules, like proteins and DNA can be made to form cystals under the right conditions.
The structure of a crystal depends on the size and chemical properties of the constituent atoms, and can take many different forms. These forms are categorized in several ways, depending on the geometry or the chemistry of the structures. The most ordered forms, i.e., the forms with highest symmetry, are cubic and hexagonal, but many variations of these structures and many other structures also exist.
Crystal structure
Crystals can be described by their structure. Crystals have translational symmetry in three dimensions - the structure looks identical after a translation over a certain distance in certain directions. This symmetry is most often defined by the shape of the unit cell, which is the smallest space-filling polyhedron which repeats in the crystal structure, or the polyhedron which is bounded by the minimal translations which show translational symmetry in each direction of symmetry.
The highest crystalline symmetry is cubic symmetry, where the crystal is identical in translation over a specific distance over any of three orthogonal directions. The unit cell is thus a cube. Conventionally, the unit cell will have atoms at each of the cube's corners, though there are some cases of cubic symmetry where this does ont occur, and it is possible to draw a unit cell where the corners are not at the location of the atoms.
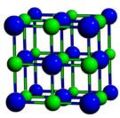
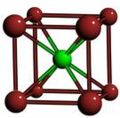
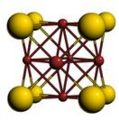
Cubic symmetry comes in a variety of forms: simple cubic, body-centered cubic, and face-centered cubic, as well as more complex forms. In body-centered cubic symmetry, the unit cell has atoms at each of the corners, and one in the center (body) of the cell. In face-centered cubic symmetry, the unit cell has atoms at each of the corners, and atoms at the center of the faces of the unit cell.
In cases where the unit cell has orthogonal axes but the length of the cell along each axis is different, the symmetry is described as orthorhombic; if two of the axes have the same unit length and the third has a different unit length, the symmetry is described as tetragonal. If the three axes of symmetry are not orthogonal - if the angle between them is not 90 degrees - they symmetry is described as triclinic, or monoclinic if one axis is at 90 degrees to the other two.
Crystals with Trigonal symmetry have three axes of symmetry in a plane, and a fourth orthogonal to them. Hexagonal symmetry occurs when the unit cell has the form of a hexagonal prism - where there are six axes of symmetry in one plane, and a further axis at 90 degrees to the other three.
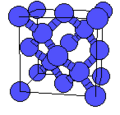
- A sample of common crystal structures at the atomic level
Crystal structure of diamond
Crystal structure of graphite
Crystal structures are not always perfect in nature, as defects can form in the structure due to impurities or stresses on the crystal. These impurities can have important effects on the mechanical and electrical properties of the crystal.
References
- Crystal Lattice Structures Web page. Center for Computational Materials Science of the United States Naval Research Laboratory (28 January 2008). Retrieved on 2008-02-07.
- Mike and Darcy Howard (1998). Introduction to Crystallography and Mineral Crystal Systems. Retrieved on 2008-02-07.