Hydrogen bond: Difference between revisions
imported>Caesar Schinas m (Bot: Update image code) |
John Leach (talk | contribs) m (Text replacement - "NMR" to "NMR spectroscopy") |
||
| Line 86: | Line 86: | ||
| doi = 10.1103/PhysRevLett.82.600 | | doi = 10.1103/PhysRevLett.82.600 | ||
| url = http://prola.aps.org/abstract/PRL/v82/i3/p600_1 | | url = http://prola.aps.org/abstract/PRL/v82/i3/p600_1 | ||
}}</ref> claimed from interpretations of the [[anisotropy|anisotropies]] in the [[Compton profile]] of ordinary ice that the hydrogen bond is partly covalent. Some [[NMR]] data on hydrogen bonds in proteins also seemed to indicate covalent bonding. These interpretations were refuted by [[Ernest R. Davidson]] and coworkers.<ref name=Ghanty2000>{{citation | }}</ref> claimed from interpretations of the [[anisotropy|anisotropies]] in the [[Compton profile]] of ordinary ice that the hydrogen bond is partly covalent. Some [[NMR spectroscopy]] data on hydrogen bonds in proteins also seemed to indicate covalent bonding. These interpretations were refuted by [[Ernest R. Davidson]] and coworkers.<ref name=Ghanty2000>{{citation | ||
| last1 = Ghanty | first1 = T.K. | | last1 = Ghanty | first1 = T.K. | ||
| last2 = Staroverov | first2 = V.N. | | last2 = Staroverov | first2 = V.N. | ||
Revision as of 16:00, 7 March 2024
In chemistry, a hydrogen bond is a type of attractive intermolecular force that exists between two partial electric charges of opposite polarity. Although stronger than most other intermolecular forces, the typical hydrogen bond is much weaker than both the ionic bond and the covalent bond. Within macromolecules such as proteins and nucleic acids, it can exist between two parts of the same molecule, and figures as an important constraint on such molecules' overall shape.
As the name "hydrogen bond" implies, one part of the bond involves a hydrogen atom. The hydrogen atom must be attached to one of the elements oxygen, nitrogen or fluorine, all of which are strongly electronegative heteroatoms. These bonding elements are known as the hydrogen-bond donor. This electronegative element attracts the electron cloud from around the hydrogen nucleus and, by decentralizing the cloud, leaves the atom with a positive partial charge. Because of the small size of hydrogen relative to other atoms and molecules, the resulting charge, though only partial, nevertheless represents a large charge density. A hydrogen bond results when this strong positive charge density attracts a lone pair of electrons on another heteroatom, which becomes the hydrogen-bond acceptor.
The hydrogen bond is not like a simple attraction between point charges, however. It possesses some degree of orientational preference, and can be shown to have some of the characteristics of a covalent bond. This covalency tends to be more extreme when acceptors bind hydrogens from more electronegative donors.
Strong covalency in a hydrogen bond raises the questions: "To which molecule or atom does the hydrogen nucleus belong?" and "Which should be labeled 'donor' and which 'acceptor'?" According to chemical convention, the donor generally is that atom to which, on separation of donor and acceptor, the retention of the hydrogen nucleus (or proton) would cause no increase in the atom's positive charge. The acceptor meanwhile is the atom or molecule that would become more positive by retaining the positively charged proton. Liquids that display hydrogen bonding are called associated liquids.
Hydrogen bonds can vary in strength from very weak (1-2 kJ mol−1) to extremely strong (40 kJ mol−1), so strong as to be indistinguishable from a covalent bond, as in the ion HF2−. Typical values include:
- O—H...:N (7 kcal/mol)
- O—H...:O (5 kcal/mol)
- N—H...:N (3 kcal/mol)
- N—H...:O (2 kcal/mol)
The length of hydrogen bonds depends on bond strength, temperature, and pressure. The bond strength itself is dependent on temperature, pressure, bond angle, and environment (usually characterized by local dielectric constant). The typical length of a hydrogen bond in water is 1.97 Å (197 pm).
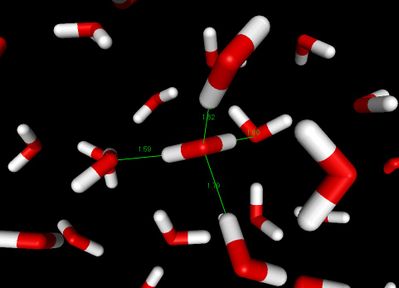
Hydrogen bond in water
The most ubiquitous, and perhaps simplest, example of a hydrogen bond is found between water molecules. In a discrete water molecule, water has two hydrogen atoms and one oxygen atom. Two molecules of water can form a hydrogen bond between them; the simplest case, when only two molecules are present, is called the water dimer and is often used as a model system. When more molecules are present, as is the case in liquid water, more bonds are possible because the oxygen of one water molecule has two lone pairs of electrons, each of which can form a hydrogen bond with hydrogens on two other water molecules. This can repeat so that every water molecule is H-bonded with up to four other molecules, as shown in the figure (two through its two lone pairs, and two through its two hydrogen atoms.)
Liquid water's high boiling point is due to the high number of hydrogen bonds each molecule can have relative to its low molecular mass. Water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. For example, hydrogen fluoride - which has three lone pairs on the F atom but only one H atom - can have a total of only two bonds (ammonia has the opposite problem: three hydrogen atoms but only one lone pair).
- H-F...H-F...H-F
The exact number of hydrogen bonds in which a molecule in liquid water participates fluctuates with time and depends on the temperature. From TIP4P liquid water simulations at 25 °C, it was estimated that each water molecule participates in an average of 3.59 hydrogen bonds. At 100 °C, this number decreases to 3.24 due to the increased molecular motion and decreased density, while at 0 °C, the average number of hydrogen bonds increases to 3.69 (Mol. Phys. 1985, 56, 1381). A more recent study (J. Chem. Phys 2005, 123, 104501) found a much smaller number of hydrogen bonds: 2.357 at 25 °C. The differences may be due to the use of a different method for defining and counting the hydrogen bonds.
Were the bond strengths more equivalent, one might instead find the atoms of two interacting water molecules partitioned into two polyatomic ions of opposite charge, specifically hydroxide (OH−) and hydronium (H3O+) (Hydronium ions are also known as 'hydroxonium' ions.)
- H-O− H3O+
Indeed, in pure water under conditions of standard temperature and pressure, this latter formulation is applicable only rarely; on average about one in every 5.5 × 108 molecules gives up a proton to another water molecule, in accordance with the value of the dissociation constant for water under such conditions. It is a crucial part of uniqueness of water
Hydrogen bonds in proteins and DNA
Hydrogen bonding also plays an important role in determining the three-dimensional structures adopted by proteins and nucleic bases. In these macromolecules, bonding between parts of the same macromolecule cause it to fold into a specific shape, which helps determine the molecule's physiological or biochemical role. The double helical structure of DNA, for example, is due largely to hydrogen bonding between the base pairs, which link one complementary strand to the other and enable replication.
In proteins, hydrogen bonds form between the backbone oxygens and amide hydrogens. When the spacing of the amino acid residues participating in a hydrogen bond occurs regularly between positions i and i + 4, an alpha helix is formed. When the spacing is less, between positions i and i + 3, then a 310 helix is formed. When two strands are joined by hydrogen bonds involving alternating residues on each participating strand, a beta sheet is formed. Hydrogen bonds also play a part in forming the tertiary structure of protein through interaction of R-groups.(See also protein structure).
A special case of intramolecular hydrogen bonds within proteins, poorly shielded from water attack and hence promoting their own dehydration, are called dehydrons.
Symmetric hydrogen bond
Symmetric hydrogen bonds have been observed recently spectroscopically in formic acid at high pressure (>GPa). Each hydrogen atom forms a partial covalent bond with two atoms rather than one. Symmetric hydrogen bonds have been postulated in ice at high pressure (ice-X). See references below (Goncharov, et al.)
Dihydrogen bond
The hydrogen bond can be compared with the closely related dihydrogen bond, which is also an intermolecular bonding interaction involving hydrogen atoms. These structures have been known for some time, and well characterized by crystallography; however, an understanding of their relationship to the conventional hydrogen bond, ionic bond, and covalent bond remains unclear. Generally, the hydrogen bond is characterized by a proton acceptor that is a lone pair of electrons in nonmetallic atoms (most notably in the nitrogen, and chalcogen groups). In some cases, these proton acceptors may be pi-bonds or metal complexes. In the dihydrogen bond, however, a metal hydride serves as a proton acceptor; thus forming a hydrogen-hydrogen interaction. Neutron diffraction has shown that the molecular geometry of these complexes are similar to hydrogen bonds, in that the bond length is very adaptable to the metal complex/hydrogen donor system.
Theory of the hydrogen bond
The mechanism of hydrogen bonding was long not well understood. However with the advent of computers and reliable computational methods, it became clear that the same contributions played a role as in other kinds of intermolecular forces. In the early 1980s it was shown that electrostatic forces are dominant in the attraction, i.e., the attractive part of the hydrogen bond is mainly due to the interaction of permanent dipoles and higher permanent multipoles of the molecules participating in the bond. In 1983, Buckingham and Fowler[1] modelled the interaction of a large number of hydrogen bonded van der Waals molecules by atomic hard sphere repulsions and distributed multipole interactions. They found a qualitative agreement between predicted and measured structures, which showed that their model accounted for the major effects of the hydrogen bonding. These results have been borne out by many quantum chemical calculations since then, of the perturbative as well as of the supermolecule type.
However, until recently some controversy persisted about the nature of the bond. A widely publicized article[2] claimed from interpretations of the anisotropies in the Compton profile of ordinary ice that the hydrogen bond is partly covalent. Some NMR spectroscopy data on hydrogen bonds in proteins also seemed to indicate covalent bonding. These interpretations were refuted by Ernest R. Davidson and coworkers.[3] It is now commonly assumed that for hydrogen bonding the same effects (exchange, electrostatic, polarization, and dispersion) play a role as for "ordinary" intermolecular forces, with electrostatics plus Pauli (hard sphere) repulsion being the most important for hydrogen bonds.
References
- ↑ Buckingham, A.D. & P.W. Fowler (1983), "Do electrostatic interactions predict structures of van der Waals molecules?", The Journal of Chemical Physics 79: 6426-6428, DOI:10.1063/1.445721
- ↑ Isaacs, E.D.; A. Shukla & P.M. Platzman et al. (1999), "Covalency of the Hydrogen Bond in Ice: A Direct X-Ray Measurement", Physical Review Letters 82 (3): 600–603, DOI:10.1103/PhysRevLett.82.600
- ↑ Ghanty, T.K.; V.N. Staroverov & P.R. Koren et al. (2000), "Is the Hydrogen Bond in Water Dimer and Ice Covalent?", Journal-American Chemical Society 122 (6): 1210–1214, DOI:10.1021/ja9937019
Some content on this page may previously have appeared on Wikipedia.