Tadalafil: Difference between revisions
imported>Caesar Schinas m (Bot: Update image code) |
mNo edit summary |
||
| Line 32: | Line 32: | ||
== References == | == References == | ||
{{cite journal|title=Molecular Biology and Pharmacology of PDE-5-Inhibitor Therapy for Erectile Dysfunction|author=J. D. Corbin and S. H. Sharron|journal=J. Androl.|volume=24|pages=S38-S41}} | {{cite journal|title=Molecular Biology and Pharmacology of PDE-5-Inhibitor Therapy for Erectile Dysfunction|author=J. D. Corbin and S. H. Sharron|journal=J. Androl.|volume=24|pages=S38-S41}} | ||
[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 17:00, 24 October 2024
|
| |||||||
| tadalafil | |||||||
| |||||||
| Uses: | Erectile Dysfunction | ||||||
| Properties: | PDE-5 inhibitor | ||||||
| Hazards: | cardiovascular risks | ||||||
| |||||||
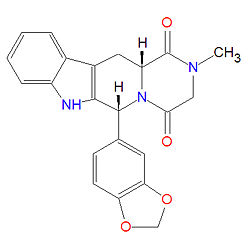
Tadalafil, commonly known by the trade name Cialis®, is a drug used to treat erectile dysfunction. It is a selective phosphodiesterase type-5 (PDE-5) inhibitor that is believed to function in the same way that sildenafil (Viagra®) and vardenafil (Levitra®) work, despite significant structural differences.
Mechanism of action
By competitively binding to PDE-5 enzymes in smooth muscle and therefore inhibiting the binding of cGMP to PDE-5, the degradation of cGMP is reduced resulting in elevated levels of cGMP in the corpus cavernosum and its supply vessels. The elevated cGMP levels relax the smooth muscles, dilate the corporeal sinusoids and increase blood flow enabling an erection. cGMP levels are normally increased during stimulation by the release of nitric oxide in the corpus cavernosum. The nitric oxide activates guanylate cyclase, an enyme, which produces cGMP. Thus, tadalafil does not enhance the normal mechanism, namely increased synthesis of cGMP, but rather reduces its degradation.
Chemistry
The chemical designation for tadalafil is pyrazino[1´,2´:1,6]pyrido[3,4-b]indole-1,4-dione, 6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6R,12aR)-. It is a crystalline solid that is practically insoluble in water and very slightly soluble in ethanol.
Drug interactions
Because tadalafil has vasodilator properties that result in decreased blood pressure, the combined use of tadalafil with other vasodilators, such as alpha-blockers, must be done cautiously. Patients with a history of heart attacks, strokes, arrythmia, hypertension, retinitis pigmentosa or currently on bosentan therapy should also be cautious.
External links
The most up-to-date information about this and other drugs can be found at the following sites. The most up-to-date information about Tadalafil and other drugs can be found at the following sites.
- Tadalafil - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Tadalafil - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Tadalafil - Detailed information from DrugBank.
References
J. D. Corbin and S. H. Sharron. "Molecular Biology and Pharmacology of PDE-5-Inhibitor Therapy for Erectile Dysfunction". J. Androl. 24: S38-S41.