Micrococcus luteus: Difference between revisions
imported>Gareth Leng |
imported>Gareth Leng |
||
| Line 21: | Line 21: | ||
==Genome structure== | ==Genome structure== | ||
''M luteus'' (NCTC2665, "Fleming strain") has one of the smallest genomes of free-living actinobacteria. It has just one circular chromosome of 2,501,097 base pairs, which is predicted to encode 2,403 proteins.<ref>Young M ''et al.'' (2010) Genome sequence of the Fleming strain of Micrococcus luteus, a simple free-living actinobacterium ''J Bacteriol'' 192:841-60. PMID 19948807</ref> The genome encodes only four sigma | ''M luteus'' (NCTC2665, "Fleming strain") has one of the smallest genomes of free-living actinobacteria. It has just one circular [[chromosome]] of 2,501,097 base pairs, which is predicted to encode 2,403 proteins.<ref>Young M ''et al.'' (2010) Genome sequence of the Fleming strain of Micrococcus luteus, a simple free-living actinobacterium ''J Bacteriol'' 192:841-60. PMID 19948807</ref> The genome encodes only four [[sigma factor]]s and 14 response regulators, a finding that may reflect its adaptation to a very narrow ecological niche (mammalian skin). ''M. luteus'' is very sensitive to [[beta-lactam antibiotic]]s, and this may reflect the presence of a reduced set of [[penicillin-binding protein]]s and the absence of a ''wblC'' gene, which has an important role in the antibiotic resistance in other actinobacteria. ''M. luteus'' has relatively few genes concerned with [[carbohydrate transport]] and metabolism, and its inability to use glucose as a sole carbon source may be because it lacks a gene encoding [[glucokinase]]. Very unusually, ''M. luteus'' seems to be able to metabolize [[glycogen]] only via [[trehalose]] and to make trehalose only via glycogen. | ||
''M. luteus'' has many interesting biological features, including its ability to show dormancy without spore formation. Although it can survive under extreme conditions, such as low temperature and starvation, ''M. luteus'' does not form spores as survival structures. Usually spores are thought of as a prerequisite for long term survival, yet ''M. luteus'' has even been resuscitated from fossilised amber.<ref>Greenblatt CL ''et al.'' (2004) Micrococcus luteus survival in amber ''Microb Ecol'' 48:120-7</ref> | ''M. luteus'' has many interesting biological features, including its ability to show dormancy without [[spore]] formation. Although it can survive under extreme conditions, such as low temperature and starvation, ''M. luteus'' does not form spores as survival structures. Usually spores are thought of as a prerequisite for long term survival, yet ''M. luteus'' has even been resuscitated from fossilised [[amber]].<ref>Greenblatt CL ''et al.'' (2004) Micrococcus luteus survival in amber ''Microb Ecol'' 48:120-7</ref> | ||
Unlike most actinobacteria, ''M. luteus'' expresses only one [[resuscitation-promoting factor]] required for emergence from dormancy, and has few other dormancy-related proteins. This resuscitation promoting factor was first described in ''M. luteus''<ref> | Unlike most actinobacteria, ''M. luteus'' expresses only one [[resuscitation-promoting factor]] required for emergence from dormancy, and has few other dormancy-related proteins. This resuscitation promoting factor was first described in ''M. luteus''<ref> | ||
Mukamolova GV ''et al.'' (2002) The rpf gene of Micrococcus luteus encodes an essential secreted growth factor ''Mol Microbiol'' 46: 611-21</ref><ref>Mukamolova GV ''et al.'' (1998) On resuscitation from the dormant state of Micrococcus luteus ''Antonie van Leeuwenhoek'' 73: 37-43</ref>, and was the founder member of a family of secreted transglycosylase-like proteins that can resuscitate bacteria from a dormant state <ref>Kell DB, Young M (2000) Bacterial dormancy and culturability: the role of autocrine growth factors ''Curr Opin Microbiol'' 3:238-43</ref><ref>Cohen-Gonsaud M ''et al.'' (2005) The structure of a resuscitation-promoting factor domain from Mycobacterium tuberculosis shows homology to lysozymes ''Nat Struct Mol Biol'' 12: 270-3</ref><ref>Mukamolova GV ''et al.'' (2006) Muralytic activity of Micrococcus luteus Rpf and its relationship to physiological activity in promoting bacterial growth and resuscitation ''Mol Microbiol'' 59: 84-98</ref> | Mukamolova GV ''et al.'' (2002) The rpf gene of Micrococcus luteus encodes an essential secreted growth factor ''Mol Microbiol'' 46: 611-21</ref><ref>Mukamolova GV ''et al.'' (1998) On resuscitation from the dormant state of Micrococcus luteus ''Antonie van Leeuwenhoek'' 73: 37-43</ref>, and was the founder member of a family of secreted transglycosylase-like proteins that can resuscitate bacteria from a dormant state <ref>Kell DB, Young M (2000) Bacterial dormancy and culturability: the role of autocrine growth factors ''Curr Opin Microbiol'' 3:238-43</ref><ref>Cohen-Gonsaud M ''et al.'' (2005) The structure of a resuscitation-promoting factor domain from Mycobacterium tuberculosis shows homology to lysozymes ''Nat Struct Mol Biol'' 12: 270-3</ref><ref>Mukamolova GV ''et al.'' (2006) Muralytic activity of Micrococcus luteus Rpf and its relationship to physiological activity in promoting bacterial growth and resuscitation ''Mol Microbiol'' 59: 84-98</ref> | ||
Revision as of 06:09, 3 December 2010
| Micrococcus Luteus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||
| Scientific classification | ||||||||||||
| ||||||||||||
| Binomial name | ||||||||||||
| Micrococcus luteus |
Micrococcus luteus (M. luteus), is a Gram-positive bacteria, 0.05 to 3.5 microns in diameter, that is most commonly found in mucous membranes such as the nasal cavities, the upper respiratory tract, and the lining of the mouth. If we were to break down the word Micrococcus, it would be as follows: Micro, for microscopic; coccus for the organism's spherical shape; luteus for "yellow". The bacteria is also found in dust, soil and the air that we breathe, and is part of the human skin flora. Although once ragarded as non-pathogenic, it is now considered an opportunistic pathogen, especially in immunocompromised patients. It is also responsible for nosocomial infections. As it is a commensal on the skin and nasal cavities, it is often overlooked as a source of clinical infection.
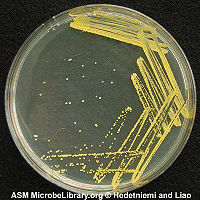
M. luteus (formerly Micrococcus lysodeikticus) is of historical interest for the part it played in Fleming’s discovery of lysozyme, to which it is exquisitely sensitive[1][2] This bacterium, which is often used for educational studies, produces bright yellow colonies on nutrient agar.
Genome structure
M luteus (NCTC2665, "Fleming strain") has one of the smallest genomes of free-living actinobacteria. It has just one circular chromosome of 2,501,097 base pairs, which is predicted to encode 2,403 proteins.[3] The genome encodes only four sigma factors and 14 response regulators, a finding that may reflect its adaptation to a very narrow ecological niche (mammalian skin). M. luteus is very sensitive to beta-lactam antibiotics, and this may reflect the presence of a reduced set of penicillin-binding proteins and the absence of a wblC gene, which has an important role in the antibiotic resistance in other actinobacteria. M. luteus has relatively few genes concerned with carbohydrate transport and metabolism, and its inability to use glucose as a sole carbon source may be because it lacks a gene encoding glucokinase. Very unusually, M. luteus seems to be able to metabolize glycogen only via trehalose and to make trehalose only via glycogen.
M. luteus has many interesting biological features, including its ability to show dormancy without spore formation. Although it can survive under extreme conditions, such as low temperature and starvation, M. luteus does not form spores as survival structures. Usually spores are thought of as a prerequisite for long term survival, yet M. luteus has even been resuscitated from fossilised amber.[4] Unlike most actinobacteria, M. luteus expresses only one resuscitation-promoting factor required for emergence from dormancy, and has few other dormancy-related proteins. This resuscitation promoting factor was first described in M. luteus[5][6], and was the founder member of a family of secreted transglycosylase-like proteins that can resuscitate bacteria from a dormant state [7][8][9]
M. luteus is capable of long-chain alkene biosynthesis (of interest for advanced biofuel production); a three-gene cluster essential for this metabolism has been identified in the genome. [10]
Cell structure and metabolism
M luteus is an obligate aerobe which can also live in very low concentrations of carbon dioxide. Its cell wall is made of peptidoglycans linked together by amino-acids which would explain its ability to absorb the dark-blue or violet stains. It is known to cause odors in humans because of its ability to break down sweat components.[11]
Ecology
M. luteus is mostly found in environments where the temperature is around 37 °C (98.6 °F) since this is the temperature at which its usual milieu is known to be (skin/nasal cavity). It also grows in beer, and is found in soil, though its lifetime is limited in soil. M. luteus is known for its growth in dairy products and can be transmitted via consumption of milk. This organism can grow on inorganic nitrogen, and cannot synthesize acid from glucose in the presence of oxygen.
M luteus has an unusual ability to tolerate and to use very toxic organic molecules as carbon sources, and metals, and can be used in the degradation of metals such as zinc, lead and nickel.[12] It has been sequenced because these features are important for potential applications in bioremediation and biotechnology[13]- these two properties are essential to dealing with toxic wastes [14] It has been found in contaminated soils, and can degrade phthalates,[15] hydrocarbons and olefinic compounds [16]
Pathology
Micrococcus is not considered as a pathogen but in individuals with a compromised immune system, such as newborn infants or patients with AIDS, M. luteus can cause skin infections that produce pruritic eruptions, sometimes with central ulceration, accompanied by severe itching; in immunocompromised patients it occasionally causes serious problems such as septic shock, pneumonia endocarditis or sepsis. [17] In hospitals, the bacteria can be transmitted by hospital staff who may have failed to wash their hands properly by going from one patient to another. The bacteria can degrade compounds in sweat into ones that produce unpleasant odors (such as foot odor). Because M. luteus is part of the natural flora of the skin and mucous membranes, when a patient has a skin infection, this bacteria is not among the first pathogens that come to mind. Some tests are needed to confirm that M. luteus is indeed causing the symptoms. Often, M. luteus can be mistaken for Staphylococcus aureus, a bacteria that, just like his homologue, can cause nosocomial infections. However, M. luteus is coagulase negative where as S. aureus is coagulase positive and is a facultative anaerobe. S. aureus is also resistant to antibiotics, particularly bacitracin.
The bacitracin susceptibility test can differentiate between M. luteus and S. aureus. For this, a blood agar plate is prepared and separated in two sections. One side is where the M. luteus will be deposited by the use of a sterile swab and the other side is where the S. aureus will be deposited. A small disk of bacitracin is then placed on each side of the blood agar plate and left to rest for a few hours. When the plate is then studied, one can notice that the area around the bacitracin disk placed in the M. luteus side of the blood agar plate is cleared out due to its antibacterial effect on the Gram-positive bacteria. This area is known as the " zone of inhibition" thus it is susceptible to bacitracin. However, the contrary is observed for the S. aureus side of the plate since such bacteria is known to be antibacterial resistant. Bacitracin finds a certain difficulty to translate Gram-negative bacteria's mRNA.
Application to Biotechnology
M. luteus has been used to study microbes’ susceptibility to lactoferrin, a glycoprotein. Lactoferrin exerts its antibacterial activities by binding to the lipomannan of the cell wall of the microbe. The protein (mostly found in mammalian [exocrine] secretion) leads to the destruction of its host in certain species. For instance, in the Micrococcus genus, the glycoprotein is effective M. luteus but not in [M. radiophilus], [M. roseus] or [M. varians]. [18]
Lactoferrin is known for its iron-binding capacities. As iron promotes microbial viability and growth, its binding to lactoferrin stops the bactericidal effect. The amount of iron present for the lactoferrin to bind plays an important role in such activity; ferric salts can stop the antibacterial effect more than the ferrous salts. Some interesting variants of M. luteus which can precipitate gold by concentrating and crystallizing it have been isolated from gold deposits in Russia. Bacteria and archaea capable of precipitating gold are now believed to have played a significant role in the biogeochemical cycling of gold, from primary mineralization in hydrothermal and deep subsurface systems to its solubilization, dispersion and re-concentration as secondary gold.[19] It has been proposed that these properties of M luteus might be used for gold adsorption and concentration from low abundance ores and depleted deposits.
References
- ↑ Fleming A (1922) On a remarkable bacteriolytic substance found in secretions and tissues Proc Roy Soc B 93:306
- ↑ Fleming A (1922) Observations on a bacteriolytic substance (Lysozyme) found in secretions and tissues Brit J Exp Path 3:252
- ↑ Young M et al. (2010) Genome sequence of the Fleming strain of Micrococcus luteus, a simple free-living actinobacterium J Bacteriol 192:841-60. PMID 19948807
- ↑ Greenblatt CL et al. (2004) Micrococcus luteus survival in amber Microb Ecol 48:120-7
- ↑ Mukamolova GV et al. (2002) The rpf gene of Micrococcus luteus encodes an essential secreted growth factor Mol Microbiol 46: 611-21
- ↑ Mukamolova GV et al. (1998) On resuscitation from the dormant state of Micrococcus luteus Antonie van Leeuwenhoek 73: 37-43
- ↑ Kell DB, Young M (2000) Bacterial dormancy and culturability: the role of autocrine growth factors Curr Opin Microbiol 3:238-43
- ↑ Cohen-Gonsaud M et al. (2005) The structure of a resuscitation-promoting factor domain from Mycobacterium tuberculosis shows homology to lysozymes Nat Struct Mol Biol 12: 270-3
- ↑ Mukamolova GV et al. (2006) Muralytic activity of Micrococcus luteus Rpf and its relationship to physiological activity in promoting bacterial growth and resuscitation Mol Microbiol 59: 84-98
- ↑ Beller HR et al. (2010) Genes involved in long-chain alkene biosynthesis in Micrococcus luteus Appl Environ Microbiol 76:1212-23 PMID 20038703
- ↑ http://microbewiki.kenyon.edu/index.php/Micrococcus
- ↑ Micrococcus luteus Tree of Life
- ↑ Micrococcus luteus NCTC 2665 Tree of Life
- ↑ Sandrin TR, Maier RM (2003) Impact of metals on the biodegradation of organic pollutants Environ Health Persp 111:1093-101
- ↑ Eaton RW (1982) Metabolism of dibutylphthalate and phthalate by Micrococcus sp. strain 12B J Bacteriol 151: 48–57
- ↑ Zhuang WQ et al. (2003) Importance of Gram-positive naphthalene-degrading bacteria in oil-contaminated tropical marine sediments Lett Appl Microbiol 36: 251
- ↑ Seifert H et al. (1995)Micrococcus luteus endocarditis: case report and review of the literature Zentralbl Bakteriol 282:431-5. PMID 9810667
- ↑ De Lillo A et al. (1997) Relationship between antibacterial activity and cell surface binding of lactoferrin in species of genus Micrococcus FEMS Microbiol Lett [ http://www3.interscience.wiley.com/cgi-bin/fulltext/119173366/main.html,ftx_abs 150:89-94
- ↑ Reith F et al. (2007)The geomicrobiology of goldISME J 1:567-84 PMID 18043665