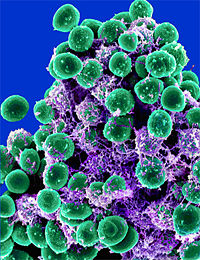
Staphylococcus aureus
Articles that lack this notice, including many Eduzendium ones, welcome your collaboration! |
| "Staphylococcus aureus" | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||
| Scientific classification | ||||||||||||
| ||||||||||||
| Species | ||||||||||||
|
Staphylococcus aureus |
Description and significance
Staphylococcus aureus is a gram-positive spherical cocci that grows in a loose, irregular cluster resembling clusters of grapes. The cluster formation is due to the cell division occuring in three planes, with the daughter cell remaining in close proximity. [1] Staphylococcus aureus may also be found singly, in pairs and in short chains of three or four cells. The bacterium can never be found in long chains. They are non-motile and non-sporing. On an ordinary medium, Staphylococcus aureus can grow within a temperature range of 10-42°C. The optimum pH ranges in between pH 7.4-7.6. The bacterium thrives best in an oxygen rich environment. S. aureus can grow on all common laboratory media such as milk, nutrient gelatin or agar. When grown on a nutrient agar and incubated for 24 hours the colonies appear to be 2-4mm in diameter.[2] The colonies appear circular, smooth, convex, shiny, and opaque.[3]
Staphylococcus aureus was first observed in 1871 by von Recklinghausen but not isolated. In 1880 Pasture obtained cultures of the bacteria and inoculated them into rabbits. Then in 1881 a surgeon by the name of Alexander Ogston documented two kinds of micrococci. The already known streptococci, arranged in chains and the other cocci arranged in clusters. Ogston named the cocci cluster Staphylococci because “Staphyle” in Greek means "bunches of grapes" and “kokakos” meaning a "berry".[4] Unfortunately, Ogston did not provide a description of the genus and therefore it was not recognized. In 1884 Rosenbach successfully isolated and grew Staphylococcus aureus from pus. Rosenbach is credited with proposing the genus Staphylococcus and the species Staphylococcus aureus.[5] He kept the genus name Staphlococcus because the bacteria was similar to that studied by Ogston. Rosenbach proposed the nomenclature for Staphylococcus aureus based on the yellow pigmentation of the colony.
In recent years the Staphylococcus aureus species has become a serious health issue. The bacteria has built up a strong resistance to treatment. By unlocking the genome sequence, researchers could understand the nature of its resistance, virulence, genetic flexibility, epidiology and physiology. Comparing the genome sequence of S. aureus with the genomes of a less virulent form and nonpathogenic species could help researchers have a better understanding of the nature of Staphylococcal aureus infections. [6]
Describe the appearance, habitat, etc. of the organism, and why it is important enough to have its genome sequenced. Describe how and where it was isolated. Include a picture or two (with sources) if you can find them.
Genome structure
The Staphylococcus aureus genome contains about 2.800 to 2.903 million base pairs of DNA. The bacteria has about 2,600 genes in its chromosome. The first whole genome sequence of S. aureus strains were completed by shot-gun random sequencing in 2001. S. aureus plasmids contains genes that encode resistance to antibiotics, heavy metals, or antiseptics.[7] Some virulence genes have been reported to be carried on plasmid, such as exfoliative toxin B and some superantigens.[8] Approximatly 75% of S. aureus genome comprises a core component of genes present in all of the strains.[9]
Describe the size and content of the genome. How many chromosomes? Circular or linear? Other interesting features? What is known about its sequence?
Does it have any plasmids? Are they important to the organism's lifestyle?
Cell structure and metabolism
Staphylococcus aureus cell wall contains a thick peptidoglycan layer and teichoic acid. The bacterium contains no flagella. Some young cultures posses microscopically visible capsules. Many noncapsulated strains of S.aureus have small amounts of capsular material on the surface.[10]
Staphylococci are facultative anaerobes that grow by aerobic respiration or by fermentation that yields lactic acid. S. aureus ferments sugars by producing acid but no gas.
Phagocytosis is a mechanism used by the host organism to combat a staphylococcal infections. S. aureus produces leukocidin, which cause the distruction of leukocytes allowing the bacteria to escape phagocytosis.[11] Leukocidin is produced in skin lesions such as boils which results in cell distruction of white blood cells and is one of the factors responsible for pus formation.
Describe any interesting features and/or cell structures; how it gains energy; what important molecules it produces.
Ecology
Staphylococcus aureus is commonly found on the skin and in various mucous membranes of man and other animals. About 20-30% of healthy people in the United States are carriers of the bacteria.[12] These individuals are usually unaware that they are carriers of the bacteria and usually never get sick from it. In hospitals the percentage is higher because of more possible contact with infected cases.
Describe any interactions with other organisms (included eukaryotes), contributions to the environment, effect on environment, etc.
Pathology
Staphylococcus aureus is one of the most common pathogenic bacteria. The organism may cause disease through tissue invasion and toxin production. The bacteria could cause a wide range of infections both internally and externally. It may cause skin infections, bone infections, pneumonia, food poisoning, Toxic shock syndrome, life threatening bloodstream infections and other serious illnesses.
Staphyolococcus aureus may cause boils usually by entering the skin through a hair folicle or a cut. They may be red, swollen and painful, and sometimes have pus. They can turn into impetigo, which turns into a crust on the skin and usually common among newborns. The organism could also cause internal abscesses. In recent years Staphylococcus aureus has become one of the leading causes of hospital acquired infections. People prone to staphylococcal infections include newborns, drug users, breastfeeding women, and people with skin disorders, surgical incisions, a weakened immune system, or chronic diseases.[13]
Extracellular and cell associated factors may influence virulence. Cell surface proteins, extracellular enzymes and toxins are present on most strains of S. aureus and increases the species ability to act as a successful pathogen.
Protein A and clumping factors are both cell surface proteins. Protien A induces platelet damage and hypersensitivity. The cocci clumps when introduced to human plasma because of this researchers use a coagulase test to help identify the bacterium. Some strains may not always test positive because they may be capsulated.
Coagulase, nucleases, lipases, hyaluronidase and protein receptors are all extracellular enzymes that play an important role in pathogenesis. The bacteria can convert fibrinogen to fibrin, has a heat stable nuclease, produces lipid hydralases which aids in infecting the skin, breaks down connective tissue, and possess receptors that facillitate adhesion to the host cell and tissue.
Toxins such as alpha hemolysin, enterotoxin, toxic shock syndrome toxin(TSST), and exfoliative (epidermolytic)toxin produced by S. aureus may cause the bacerium to be more virulent. Alpha hemolysin is a protein that is inactivated at 70oC but activated at 100oC. It is toxic to macrophages, lysosomes, muscle tissues, renal cortex, and the circulatory system. Enterotoxin is also a superantigen responsible for causing food poisoning which may lead to nausea, vomiting, and diarrhea. Toxic shock syndrome toxin is a superantigen as well and causes toxic shock syndrome in the infected host. It may prove to be a potentially fatal multisystem disease. The infected host can experiance a fever, hypotension, myalgia, vomiting, diarrhea and mucosal hyperemia. The exfoliative (epidermolytic)toxin is responsible for staphycoccal scalded skin syndrome(SSSS). It is an exfoliative skin disease which causes the outer layer of the epidermis to be separated from the underlying tissues. Symptoms associated with the disease are a fever, malaise and irritability following an upper respiratory infection.
Staphylococcus aureus has a high incidence of drug resistance with methicillin-resistant strains resistant to ß-lactams and most other antibiotics. Infections are enhanced in the presence of foreign materials inside the body such as tampons, surgical packing or intravenous catheters.
How does this organism cause disease? Human, animal, plant hosts? Virulence factors, as well as patient symptoms.
Application to Biotechnology
In an anaerobic environment Staphylococcus aureus can reduce mannitol to lactic acid, which differentiates it from other species of staphylococci. The bacterium is the only coagulase positive and ß-hemolytic staphylococcus. When grown on a nutrient agar containing phenolphtalein diphosphate the bacterium produces phosphatase. When ammonia vapor is introduced to the culture, the colonies assume a bright pink color due to the presence of free phenophalein. Researchers use this test to distinguish between Staphylococcus aureus from S. epidermidis.
Does this organism produce any useful compounds or enzymes? What are they and how are they used?
Current Research
Enter summaries of the most recent research here--at least three required
Methicillin-resistant staphylococcus aureus
Methicillin-resistant staphylococcus aureus (MRSA) is a variety of staphylococcus that is resistant to commonly used antibiotics such as methicillin. MRSA is predominantly a nosocomial pathogen causing hospital aquired infections as well as community aquired infections.[9]
Screening for MRSA
Studies are conflicting whether screening patients upon admittance to the hospital reduces nosocomial MRSA infections. A study of surgical patients was negative[14], while a study was that screened all admissions was positive.[15] In the positive study the patients received "5-day regimen comprising mupirocin calcium, 2% twice daily to the nares, and a chlorhexidine 4% wash or shower every 2 days" while in the negative study, patients who were found to have MRSA received "nasal mupirocin ointment and chlorhexidine body washing" without further details provided.
Eradication of MRSA
Numerous studies have looked at the role of decolonization to stop carriage.[16][17][18][19][20][21][22]
Whole body washing alone does not seem sufficient to reduce carriage.[23] Intranasal mupirocin with chlorhexidine soap body washing does not always suffice.mupirocin (group M) or placebo (group P) applied to the anterior nares for 5 days; both groups used chlorhexidine soap for body washing. Mupirocin alone may not work, especially in long-term care facilities[24] or military recruits[25].
A meta-analysis by the Cochrane Collaboration was inconclusive.[26]
References
- ↑ Textbook of Microbiology
- ↑ Textbook of Microbiology
- ↑ Textbook of Microbiology
- ↑ Medicinenet: Staphylococcus aureus
- ↑ Textbook of Microbiology
- ↑ [2]
- ↑ Insights on Virulence and Antibiotic Resistance: A Review of the Accessory Genome of Staphylococcus aureus
- ↑ Yamaguchi T, Hayashi T, Takami H, et al. Complete nucleotide sequence of a Staphylococcus aureus exfoliative toxin B plasmid and identification of a novel ADPribosyltransferase, EDIN-C. Infect Immun. 2001;69(12):7760-7771.
- ↑ Lindsay JA, Holden MTG. Understanding the rise of the superbug: investigation of the evolution and genomic variation of Staphylococcus aureus. Funct Integr Genomics. 2006;6(3):186-201
- ↑ Textbook of Microbiology
- ↑ Brock, Madigan, Martinko, Parker. Biology of Microorganisms New Jersey: Prentice Hall, 1994.
- ↑ Medicinenet: Staphylococcus aureus
- ↑ Merck: Staphylococcal Infections
- ↑ Harbarth S, Fankhauser C, Schrenzel J, et al (2008). "Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients". JAMA 299 (10): 1149-57. DOI:10.1001/jama.299.10.1149. PMID 18334690. Research Blogging.
- ↑ Robicsek A, Beaumont JL, Paule SM, et al (2008). "Universal Surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals". Ann. Intern. Med. 148 (6): 409-18. PMID 18347349. [e]
- ↑ Watanakunakorn C, Axelson C, Bota B, Stahl C (1995). "Mupirocin ointment with and without chlorhexidine baths in the eradication of Staphylococcus aureus nasal carriage in nursing home residents". Am J Infect Control 23 (5): 306–9. PMID 8585642. [e]
- ↑ Simor AE, Phillips E, McGeer A, et al (2007). "Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization". Clin. Infect. Dis. 44 (2): 178–85. DOI:10.1086/510392. PMID 17173213. Research Blogging.
- ↑ Rohr U, Mueller C, Wilhelm M, Muhr G, Gatermann S (2003). "Methicillin-resistant Staphylococcus aureus whole-body decolonization among hospitalized patients with variable site colonization by using mupirocin in combination with octenidine dihydrochloride". J. Hosp. Infect. 54 (4): 305–9. PMID 12919762. [e]
- ↑ Sandri AM, Dalarosa MG, Ruschel de Alcantara L, da Silva Elias L, Zavascki AP (2006). "Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA". Infect Control Hosp Epidemiol 27 (2): 185–7. DOI:10.1086/500625. PMID 16465636. Research Blogging.
- ↑ Dupeyron C, Campillo B, Bordes M, Faubert E, Richardet JP, Mangeney N (2002). "A clinical trial of mupirocin in the eradication of methicillin-resistant Staphylococcus aureus nasal carriage in a digestive disease unit". J. Hosp. Infect. 52 (4): 281–7. PMID 12473473. [e]
- ↑ Walsh TJ, Standiford HC, Reboli AC, et al (1993). "Randomized double-blinded trial of rifampin with either novobiocin or trimethoprim-sulfamethoxazole against methicillin-resistant Staphylococcus aureus colonization: prevention of antimicrobial resistance and effect of host factors on outcome". Antimicrob. Agents Chemother. 37 (6): 1334–42. PMID 8328783. [e]
- ↑ Ridenour G, Lampen R, Federspiel J, Kritchevsky S, Wong E, Climo M (2007). "Selective use of intranasal mupirocin and chlorhexidine bathing and the incidence of methicillin-resistant Staphylococcus aureus colonization and infection among intensive care unit patients". Infect Control Hosp Epidemiol 28 (10): 1155–61. DOI:10.1086/520102. PMID 17828692. Research Blogging.
- ↑ Wendt C, Schinke S, Württemberger M, Oberdorfer K, Bock-Hensley O, von Baum H (2007). "Value of whole-body washing with chlorhexidine for the eradication of methicillin-resistant Staphylococcus aureus: a randomized, placebo-controlled, double-blind clinical trial". Infect Control Hosp Epidemiol 28 (9): 1036–43. DOI:10.1086/519929. PMID 17932823. Research Blogging.
- ↑ Kauffman CA, Terpenning MS, He X, et al (1993). "Attempts to eradicate methicillin-resistant Staphylococcus aureus from a long-term-care facility with the use of mupirocin ointment". Am. J. Med. 94 (4): 371–8. PMID 8475930. [e]
- ↑ Ellis MW, Griffith ME, Dooley DP, et al (2007). "Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial". Antimicrob. Agents Chemother. 51 (10): 3591–8. DOI:10.1128/AAC.01086-06. PMID 17682105. Research Blogging.
- ↑ Loeb M, Main C, Walker-Dilks C, Eady A (2003). "Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization". Cochrane Database Syst Rev (4): CD003340. DOI:10.1002/14651858.CD003340. PMID 14583969. Research Blogging.
http://www.nih.org/NIHnewWebsite/nihPublicHealth/pdfs/MRSAParentsGuide.pdf