Bariatric surgery
Currently bariatric surgery is the most consistently effective treatment for obesity. Long-term studies show that this treatment can produce significant and sustained reduction in body weight, recovery from diabetes mellitus type 2, improvement in cardiovascular risk factors, and a reduction in mortality of 23% from 40%.[1] [2][3]:The U.S. National Institutes of Health recommends bariatric surgery for people with a body mass index (BMI) of > 40, and for people with BMI of > 35 who have serious coexisting medical conditions such as diabetes. This article discusses the specific bariatric operations and report how surgery affects hormonal modulation of both the gut-brain and adipose-brain axes.
For many severely obese patients, bariatric surgery has proved to be the only effective method in the long term treatment of obesity. Bariatric surgery works by altering the anatomy of the gastrointestinal tract. This limits the volume of food the stomach can hold, interferes with the absorption of calories, and interferes with orexigenic and anorexigenic signalling between the gut, adipose tissues and the brain. Bariatric surgical procedures are continually evolving, and some of the procedures currently in use are described here.
Restrictive Surgery
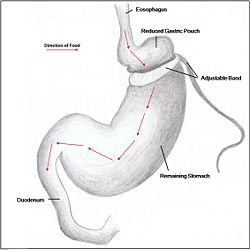
Restrictive surgery reduces the size of the stomach causing decreased hunger, and increased satiety following ingestion of food. Surgical procedures include gastric banding (Fig. 1), where an adjustable band is clipped just below the cardia of the stomach creating a small gastric pouch above. Other forms of restrictive surgery include gastroplasty, also called 'stomach stapling', and sleeve gastrectomy, where a large part of the stomach is removed.
Malabsorptive Surgery
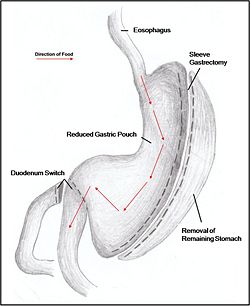
Jejuno-ileal bypass, duodenojejunal bypass and biliopancreatic bypass have all been performed to reduce the amount of calories absorbed by reducing the size of the stomach. However, as a result of extreme malnourishment, these procedures are now rarely performed. Instead, duodenal switches are the dominant form of malabsorptive surgery.
Combination Surgery
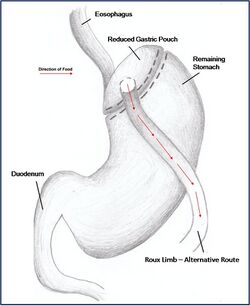
Combination surgery combines the benefits of both restrictive and malabsorptive surgery. The most common bariatric procedure in the USA is the Roux-en-Y Gastric Bypass (Fig. 2). Most restrictive procedures are performed in conjunction with a duodenum switch. For instance, a sleeve gastrectomy reduces the stomach size drastically while the additional switch of the duodenum reduces the amount of calories absorbed (Fig. 3).
The Efficacy of Surgery
There is substantial weight loss following bariatric surgery. A meta-analysis of studies of 10,172 patients reported that, on average, patients lose 61% of their weight; bilopancreatic diversion and duodenal switch procedures produce most weight loss (70% weight loss), followed closely by gastroplasty (68.2%) and gastric bypass (61.6%), with gastric banding ranked lowest (48%). [4] Bariatric surgery also improves several obesity related comorbidities which may prove to be of greater benefit than weight loss to the individual.
- An improvement or resolution of type 2 diabetes or impaired glucose tolerance was found following all types of bariatric surgeries. A resolution of diabetes allows patients to end their diabetes associated medication and improved glucose tolerance allows patients to maintain normal levels of blood glucose. For patients with diabetes before surgery, 77% resolved and 86% improved their diabetes with biliopancreatic diversion and duodenal switch procedures ranking highest (99% resolution), followed by gastric bypass (84%), gastroplasty (72%), and lastly gastric banding (48%).
- [Hyperlipidemia]] (high levels of lipids or lipoproteins in the blood), hypercholesterolemia (high blood cholesterol) and hypertriglyceridemia (high levels of triglycerides in blood) also improved following all bariatric surgeries, with reported improvements of 70% or better. Greatest improvements are seen following biliopancreatic diversion, duodenal switch and gastric bypass procedures.
- Hypertension (high blood pressure) also improved following all bariatric surgeries. A meta-analysis reported that 79% of patients show improvement in their hypertension and, of 4,805 patients studied, 62% resolved their condition. The efficacy rankings of each surgery procedure vary in terms of improvement or resolution.
- Obstructive sleep apnea (a sleep disorder characterised by pauses in breathing caused by obstructed airways) and obesity hypoventilation syndrome improved following all bariatric procedures, with 86% of patients resolving their sleep apnea.
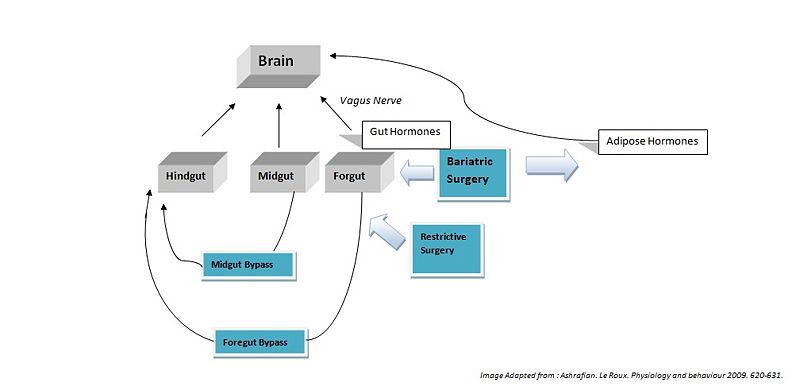
Surgical Effects on Gut Hormones
Hindgut Hormones[2]
Peptide tyrosine tyrosine (PYY) is a peptide belonging to the neuropeptide Y (NPY) family. PYY is normally released in proportion to calorie intake and has recently been described as an important feedback molecule in the Gut-Brain axis. As well as being a satiety signal, PPY has also been suggested to increase energy expenditure. After bypass and restrictive surgery, basal and postprandial PYY levels increase [5]. In addition, basal PYY levels increase after ileal resection, and patients have been reported to have lost weight [6]. By contrast, diet-induced weight loss did not increase PYY levels [7].
Glucagon-like peptide-1 (GLP-1), like PYY, as a circulating peptide hormone that acts on the hypothalamus as an anorexigenic signal. Most patients show a marked increase in basal and postprandial concentrations of GLP-1 after foregut bypass surgery. Midgut bypass also increases GLP-1 secretion, but studies on restrictive surgery have shown either no change or a decrease in basal levels of GLP-1. Of specific interest GLP-1 has been shown to improve postprandial glycaemic control, being a potent incretin mimetic. A GLP-1 agonist, exenatide, has not yet been approved for use as an anti-obesity drug, but is being used to treat type 2 diabetes mellitus <refname=Ashrafian />
Midgut Hormones[3]
Neurotensin (NT), is an anorexigenic signal whose expression is downregulated in ob/ob (leptin deficient) mice. Following bypass surgeries (5 of 6 reported) an increase in NT levels has been demonstrated which could contribute to the anorexigenic effects (Ashrafian H, le Roux CW, 2009).
Foregut Hormones[4]
Ghrelin is an orexigenic hormone released from the fundus of the stomach. As a result, ghrelin levels increase when calorie intake is reduced during dieting. However, of 42 studies following foregut bypass surgeries, only 50% reported a decrease in ghrelin release. Many of these studies reported ghrelin levels immediately after surgery, whereas studies of long term ghrelin change following surgery show no significant trend. Results for restrictive surgeries show only an 18% decrease in circulating concentrations of ghrelin. [8] [9].
Cholecystokinin (CKK) was the first anorexigenic gut peptide to be implicated in the control of appetite [10]. However, studies suggest that it plays no role in the changes in appetite following bariatric surgery [5]
Glucose-dependent Insulinotrophic Peptide (GIP) is a hormone produced in the foregut, and released when simple, energy-dense nutrients like glucose and free fatty acids are present. and it promotes nutrient (energy) storage [11]. Those with obesity and diabetes often have a diet-induced, chronic elevation in GIP levels with peripheral receptor down-regulation that is analogous to insulin insensitivity. Diet-induced weight loss increases GIP levels after a nutrient-rich meal and prevents further weight loss because the body counters the perceived starvation by increasing nutrient deposition in central and peripheral stores. By contrast, proximal foregut bypass surgery, roux-en-Y gastric bypass and biliopancreatic diversion with duodenal switch decrease GIP levels.
Surgical Treatment as Opposed to Diet
It is still not clear how bariatric surgery works, but it is thought to increase energy metabolism, reduce orexigenic signals, increase anorexigenic signals and to interfere with calorie absorption. After diet-induced weight loss, orexigenic hormones like ghrelin are released in abundance as the body is in 'starvation mode' - increasing the chances of the individual to over-eat to regain energy therefore regaining the weight. However, although bariatric surgery similarly induces weight loss - the reduction in stomach size allows a proportionate decrease in ghrelin released. Hence, although there is reduced calorie intake, less orexigenic signals are released.[8]
Under fasting conditions, most obese people have lower circulating concentrations of PYY than those who are lean. A year after bariatric surgery, such as jejunoileal bypass and vertical banding, the levels of fasting PYY are increased. Moreover, concentrations within an hour of eating did not seem to change considerably - whether in an obese, lean, or postoperative. However, after Roux-en-Y gastric bypass, there is a postprandial PYY surge. It is believed that the reason for this is the removal of the pylorus (as well as a portion of the stomach) that allows food to enter the small intestine more quickly. This early rise in PYY levels will therefore also induce satiety early. Again, although such cases have been reported, controversy still surrounds the exact mechanisms and effects of surgery on PYY levels due to the variability between individuals.
Type 2 diabetes develops in about 41% of obese patients - the correlative link is commonly termed Diabesity. Diet-induced weight loss ameliorates the insulin response but not to the extent where euglycemia is restored. Furthermore, when weight is re-gained after diets it is often accompanied by the re-establishment of insulin resistance. After biliopancreatic diversion with Roux-en-Y gastric bypass, insulin sensitivity improves. Within a year after bariatric surgery, despite the weight loss achieved, patients are usually still obese (with a BMI of ~30 kg/m2), but improvements of insulin resistance occur a few months after the operation. Therefore, the initial improvement of type 2 diabetes following bariatric surgery seems to take place while the patient is still obese.[12].
Adverse effects of surgery
Clinical practice guidelines address avoiding adverse effects after surgery.[13] Recommendations include:
- Patients should consume 60 to 120 g of protein per day
- Patients should take vitamin and mineral supplementation
- For patients who underwent a malabsorptive procedure
- Blood levels of vitamin D, calcium, phosphorus, parathyroid hormone, and alkaline phosphatase should be measured every 6 months and bone density should be measured yearly until stable
- Blood levels of ferritin , vitamin B12, and folate should be measured every 6 months for 2 years, and then annually.
Complications
Complications from weight loss surgery are frequent.[14] The reduction in intestinal absorptive capacity may cause, for instance, persistent anemia only reversible though the intravenous route.[15] Although perioperative mortality may be low in selected surgical centers[16], perioperative mortality is higher in unselected centers[17], especially if the surgeons has done less than 35 procedures in the last 6 years.[17]
A clinical prediction rule can estimate complications.[18][19] In this rule, 1 point is given for each of 5 preoperative variables:
- age >=45 years
- male gender
- body mass index >=50 kg/m2
- hypertension
- risk factors for pulmonary embolism (previous thromboembolism, preoperative vena cava filter, hypoventilation, pulmonary hypertension)
Based on the number of points, risk classfication is:
| OS-MRS Class | Points | Mortality |
|---|---|---|
| A | 0 - 1 | 0.2% to 0.3% |
| B | 2 - 3 | 1.2% to 1.9% |
| C | 4 - 5 | 2.4% to 7.6% |
References
- ↑ Robinson MK (2009) Editorial, Surgical treatment of obesity -- weighing the facts N Engl J Med 361:520
- ↑ Snow V et al. (2005). "Pharmacologic and surgical management of obesity in primary care: a clinical practice guideline from the American College of Physicians". Ann Intern Med. 142: 525–31. PMID 15809464. [e]
- ↑ Maggard MA et al. (2005). "Meta-analysis: surgical treatment of obesity". Ann Intern Med 142: 547–59. PMID 15809466. [e]
- ↑ Buchwald H et al. (2004) Bariatric surgery: A systematic review and meta-analysis JAMA 292:1724-37
- ↑ 5.0 5.1 Ashrafian H, le Roux CW (2009) Metabolic surgery and gut hormones - A review of bariatric entero-humoral modulation Physiol Behav 97:620-31 [1]
- ↑ Broberger C (2005) Brain Regulation of food intake and appetite: molecules and networks J Int Med 258:301-27
- ↑ Olivan B et al. (2009) Effect of weight loss by diet or gastric bypass Surgery on peptide YY3-36 levels. Ann Surg 249:948-53
- ↑ 8.0 8.1 Korner J et al. (2005) Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 90:359-65
- ↑ Holdstock C et al.(2003) Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab 88:3177-83
- ↑ (murphy)
- ↑ (Rosen, 2009)
- ↑ Polyzogopoulou EV et al. (2003) Restoration of euglycaemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery Diabetes 52:1098-103
- ↑ Endocrine Society (2010) Management of the Post-Bariatric Surgery Patient. Journal of Clinical Endocrinology & Metabolism
- ↑ Encinosa WE et al. (2006). "Healthcare utilization and outcomes after bariatric surgery". Medical Care 44: 706-12. DOI:10.1097/01.mlr.0000220833.89050.ed. PMID 16862031. Research Blogging.
- ↑ Mizón C et al. (2007). "Persistent anemia after Roux-en-Y gastric bypass". Nutrition 23: 277–80. PMID 17352964.
- ↑ The longitudinal assessment of bariatric surgery (LABS) consortium. "Perioperative safety in the longitudinal assessment of bariatric surgery". N Engl J Med 361: 445-54. PMID 19641201.
- ↑ 17.0 17.1 Flum DR et al. (2005). "Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures". JAMA 294: 1903–8. PMID 16234496.
- ↑ 18.0 18.1 DeMaria EJ et al. (2007). "Validation of the obesity surgery mortality risk score in a multicenter study proves it stratifies mortality risk in patients undergoing gastric bypass for morbid obesity.". Ann Surg 246: 578-82; discussion 583-4. PMID 17893494.
- ↑ 19.0 19.1 DeMaria EJ et al. (2007). "Obesity surgery mortality risk score: proposal for a clinically useful score to predict mortality risk in patients undergoing gastric bypass". Surg Obes Relat Dis 3: 134-40. DOI:10.1016/j.soard.2007.01.005. PMID 17386394. Research Blogging.